More Information
Submitted: January 19, 2022 | Approved: March 25, 2022 | Published: March 26, 2022
How to cite this article: Ehsanifar M, Rafati M, Yavari Z. Indoor air pollution and behavioral factors affecting to COVID-19 transition. J Community Med Health Solut. 2022; 3: 016-020.
DOI: 10.29328/journal.jcmhs.1001015
Copyright License: © 2022 Ehsanifar M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Indoor environment; COVID-19 transition; Airborne SARS-CoV-2; Behavioral factors; Indoor Air pollution
Indoor air pollution and behavioral factors affecting to COVID-19 transition
Mojtaba Ehsanifar1,2*, Mehravar Rafati3 and Zeinab Yavari4
1Department of Environmental Health Engineering, School of Public Health, Iran University of Medical Sciences, Tehran, Iran
2Anatomical Sciences Research Center, Kashan University of Medical Sciences, Kashan, Iran
3Department of Medical Physics and Radiology, Faculty of Paramedicine, Kashan University of Medical Sciences, Kashan, Iran
4Department of Civil and Architectural Engineering, Sultan Qaboos University, Muscat, Oman
*Address for Correspondence: Dr. Mojtaba Ehsanifar, Department of Environmental Health Engineering, School of Public Health, Iran University of Medical Sciences, Tehran, Iran, Email: [email protected]
Environmental and behavioral factors are very important for exposure to airborne SARS-CoV-2. Indoor environments are related to infection events, including super-spreader events and outbreaks. Indoor, poorly ventilated, and crowded areas, such as restaurants, cinemas, and bars can be effective in the accumulation of aerosols full of viruses, especially if people are in conversations and stay there for a long time period. At longer distances (more than 1.5 meters), small aerosols that can stay in the air for a longer period of time are dominant. The super-spreader events in which people have been infected at a distance away show that this remote transmission occurs. The exposure risk to longer intervals is likely to be more in domestic environments and indoor spaces that lack sufficient ventilation. Layer interventions are of fundamental importance. Therefore, it is important to take preventive measures as much as possible and follow them as carefully as possible, because no intervention alone will be effective in eliminating the risk. These include spacing, lining, hand hygiene, filtration, and ventilation.
A review of epidemiological studies of SARS-CoV-2 transmission has shown that the COVID-19 prevalence from the point source (an infected person transmits it to others) often occurs in people who are for a long time at home or in crowded environments are exposed to it (e.g., correctional facilities, bars, long-term care facilities, and homeless shelters). By examining these epidemiological patterns, experts become aware of how SARS-CoV-2 is transmitted [1]. Epidemiologists cite basic reproduction numbers, to describe the spread of the virus, known as the R0 or “R naught” also. This value indicates an average number of immunologically naive individuals that are infected by an infectious person [2].
It is important to recognize that R0 varies over time and conditions and is not absolute. An important challenge for scientists is understanding the “super-spreading events” [3]. Also, another study shows airborne transmission [4]. Epidemiological findings also show that the secondary attack rate the probability of infection occurring in susceptible individuals in a particular group - is generally higher among home contacts. This risk seems to be low among the other interpersonal contacts, for example talking and sharing food and even other interactions such as shopping. Studies show that a higher percentage of people infected with the SARS-CoV-2 had eaten in the restaurant (where the mask is removed to eat) 14 days before COVID-19 onset [1,5]. Also, the findings show that mask-wearing slows down the spread of the virus in the community, and the daily cases number in the states where the mask is enforced is much lower. Droplets and air transfer are inseparable [6]. In general, a pattern for classifying types of airborne transmission includes obligate transfer (infection transmission occurs only through aerosol particles deposition), preferential transmission (dominant transmission through aerosol particles deposition and state-dependent clinical manifestations), and opportunistic transfer (although transfer non-airborne is the most common, under optimal conditions aerosol particles may transmit the infection) is provided [7]. There are some environmental factors and variables (e.g. air characteristics, lack of protective measures such as face cover and mask use, constant exposure during the infectious period) which can affect the relative importance of droplets against airborne infection transmission. This relative importance is an issue that needs to be addressed by public health officials as they seek to limit the transmission of the disease and reduce the unhealthy effects of the epidemic. The relationship between environment, agent, and the host is shown by an epidemiological triangle. External environment or factors causes or allow disease transmission, the microbe or agent causes the disease, and the host or organism has the disease [4,6]. More study is necessary to determine adequate and appropriate ventilation to minimize exposure to infection, especially in crowded places and indoors.
Can people inhale SARS-CoV-2 enough to infect?
The R-value (value of R, or effective reproductive number) is considered in association whit the droplets’ relative importance versus aerosol particles transmission. The SARS-CoV-2 has a longer incubation period and the highest average R0. Although the comparison shows the challenge of SARS-CoV-2 in a high proportion of individuals who are simultaneously infectious and asymptomatic, the reproductive number can vary over time. Was highlighted need to consider variability in R0 in one systematic review [2] of measles R0 estimates over time and based on geography, where it was indicated that R0 estimates are wider than 12-18, which is often with SARS-CoV-2 compared and does not reflect the conditions of the affected population.
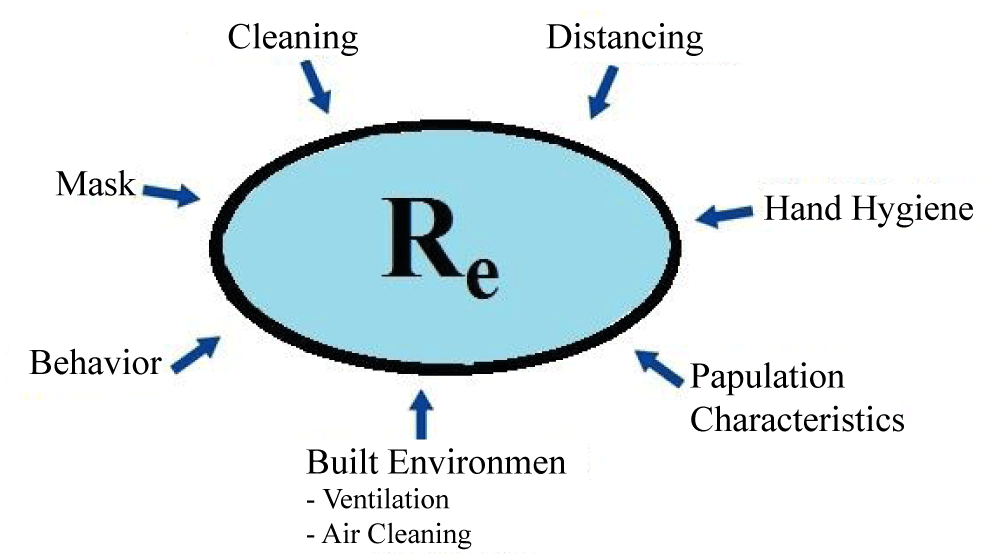
Given the limitations of R0, the concept of an effective reproductive number [2,8] (called Rt or Re), which describes transmission as soon as an epidemic occurs, depends on environmental factors which contribute to transmission [2]. Some of the factors that may contribute to this value include cleanliness, personal behavior, demographic characteristics, mask, and others (Figure 1). Factors affecting the transfer can be considered as environmental factors at the micro and macro levels. At the micro-level, the factors include the ability to work from the home and some factors such as the built environment characteristics and the amount of ventilation in the buildings. At the macro level, the factors are driven by the national and the local policies, such as staying home, public health, or masking instructions.
Figure 1: This figure indicates factors that can influence the Re. (Re (or Rt), Effective Reproduction Number, describing the transmission once the epidemic is established).
Investigating the relationship between disease and infectious dose, how exposure, and how the biological modifiers for example sex, age, and medical background conditions can affect disease outcomes are important. Animal and human studies on coronaviruses of different viruses have shown a range of infectious doses which depend on external factors [6]. Initial attempts to determine the amount of virus required to infect 50% of exposed individuals or infectious dose 50 (ID50) based on the human tests or natural events of other viruses; ID50 varies from 10 to 1000 infectious virions. According to studies, in the case of SARS-CoV-2, the ID50 value is less than 1000 infectious viruses transmitted via intranasal drops [9]. The difference between aerosols and droplets is important, and the factors affecting infectious dose are also important. For influenza, micron-sized aerosol particles were found to require much lower doses of infection than large droplets [10]. In addition, the level of recipient immunity is important, a study of H1N1 influenza indicated in people that with pre-existing immunity the ID50 increased to 383,000 virions [11]. Other factors that affect infectious dose include sex, age, distribution, and receptor abundance. SARS-CoV-2 to angiotensin-converting enzyme receptors binding, and its number and distribution depending on sex, age, and individual behaviors and habits such as smoking are vary greatly [12]. SARS-CoV-2 infection can occur even at low doses. The SARS-CoV-2 virus is transmitted through aerosol particles in animal models. SARS-CoV-2 transmission studies in cats caged together and mice caged together have shown that direct or close contact is a more effective method of transmission [13-16]. In hamsters, aerosol transfer was the predominant mode. Time is also important; Hamsters were not able to the infection transmit for more than 6 days after being infected [17]. Therefore, the transmission dominant method can be different for animal models, and between others, the transferable period is short.
Dosage, as well as individual characteristics, is important factors in determining the severity of SARS-CoV-2 disease
In mice and hamsters, the higher dose, the more severe the disease for coronaviruses, such as SARS-CoV-2 [9,18]. However, the severity of the disease varies depending on the host condition, including genetic background, age, and health status, even at the same dose. The genetic differences change disease severity even independently of the viral dose in mice [19]. The same is true of obesity in mice. Aging also is a significant risk factor for individuals and some studies have shown that the severity of the disease increases with age. With age, our innate and acquired immune responses to the infection, such as particles mucociliary clearing from lungs, decrease. While gender, underlying health conditions, age, and genetics can all affect the severity of the disease, these conditions combined do not always lead to a predictable outcome. Dosage affects the disease severity, but this is not the only variable that leads to the outcome. Also, sex as a main biological variable may change the COVID-19 outcome. According to global reports, the male mortality rate is higher regardless of the national mortality rate. Have been shown that males excrete more viruses during a longer time. One of the reasons for the higher risk of the disease in men can be the sex differences in the entry of the virus and the immune response [20,21]. Such gender differences with other pathogens have also been observed, with young males transmitting TB bacilli more often than women, children, or older men. The importance of airborne aerosol particles in the transmission of SARS-CoV-2, which is amplified by animal models, is related to the severity of the disease, the infectious dose, and is influenced by underlying health status and genetic factors such as age, and sex [3,5].
Wearing a mask (face masks) reduces both the emission of droplets and the absorption of airborne particles
Indoor ventilation reduces exposure to aerosol particles. There is no rate of “one-size-fits-all” to eliminate the risk of exposure and ventilation should be based on the occupancy of the indoor environment. The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), for example, recommend 6.7 L/s per person (L/S pp) ventilation rates for the school classrooms and 2.5 L/s pp for the churches (ASHRAE 2003). A study of university dormitories showed that a ventilation rate of fewer than 5 L/s pp can affect acute respiratory infections [22]. The other research has suggested a much higher ventilation rate. One study, for example, found that a rate of outdoor air supply of fewer than 25 L/s pp increased the risk of decreased productivity, symptoms of sick building syndrome, and increased short-term sick leave [23].
A study of the prevalence of COVID-19 in a restaurant in Guangzhou, China, showed that lack of ventilation contributed to the outbreak of COVID-19.
It is possible that the lack of fresh air from outside and the presence of strong airflow from the air conditioner in just only one location is thought to have played a role in the disease prevalence [24]. Ventilation lack was also involved in the prevalence of the Skagit Valley Chorale Rehearsal prevalence. The facility where training took place probably had less than 1 air change per hour, which allowed a significant accumulation of airborne aerosol particles during 2.5-hour practice [25]. Research is necessary to determine what rates of ventilation to control infectious diseases in the air are warranted. Current practices are not conducive to minimizing energy consumption for high ventilation, so the future challenge will be to develop cost-effective and efficient strategies and technologies that also provide high ventilation rates.
Continuous reduction of disease transmission follow-ing the use of face coverings (mask)
In experimental studies of the flu in the 1960s, the dose of aerosol infection was far lower than drops [26]. Despite the difficulty in separating airborne particles from droplets in samples, the biological transmission of the disease through the air has been confirmed. This is further corroborated by studies demonstrating that viruses may remain infectious on the air and surfaces for hours [26,27]. Typical room ventilation alone cannot reduce the effect of shared respiratory plumes exposure when individuals are close to each other. Transmission of the virus at shorter distances seems to be the dominant route for the spread of SARS-CoV-2, so social distancing is the effective way for limiting the spread of disease [28]. It is indicated that face-coverings or masks reduce aerosol particles and droplets emission at the source by 52% to 90%, depending on the type of mask, fit and use. Even a reduction of 10% in the virus transmission is valuable, and the negative result in this test does not mean that the masks do not good work. Wear masks also reduce the wearer’s intake of droplets and aerosol particles by 25% to 90%, depending on the details. In addition, wearing the masks reduce the jet emission of respiratory plumes and limit the interval traveled by aerosol particles and droplets [29]. Wearing masks reduces both the emission and the intake of airborne aerosol particles and droplets by the wearer. Surgical masks have been estimated to reduce the influenza aerosols emissions from infected peoples by 67% to 75% and intake by 50% to 83% [30]. For smaller aerosol (< 5 μm) such as larger droplets and aerosols containing coronaviruses, the surgical masks were effective in controlling the source as well as protecting the wearer [31]. Based on the results of studies from the other respiratory viruses, findings show a 10% to 20% reduction in transmission through a combination of increased hand hygiene and mask use [32]. In general, there is clear mechanical evidence that face cover and mask protect the wearer because we know that not all face covers and masks are made equal and that there are different levels of protection masks that are afforded. As mentioned, global masking alone cannot be sufficient to prevent infectious spread. The disease’s largest outbreaks were observed in communities where individuals do not wear face masks, but two community epidemics have occurred in Hong Kong that despite 99% of masks being used in required public places (for example taxis and mass transit, but not in the social environments as well as restaurants or bars) that indicate need to layer by the other methods, such as social distancing [29]. Current evidence indicates that SARS-CoV-2 transmission can occur from multiple pathways that support the need for layered interventions. The masks restrict the bidirectional transfer of aerosol particles and infectious droplets and protect the mask wearer and also those surrounding them. Face shields and Plexiglas barriers reduce droplet transport but do not restrict aerosol particles as they are transported through currents of air. Further measurements are also needed on how generated the size of aerosol particles and droplets based on activities [4]. Indoor activities, even with the wear masks, should be modified and limited and individual distances should be maintained due to the local leakage from masks, the accumulation of indoor aerosol particles, and considering the respiratory zone importance in addition to the overall flow of indoor air. Filtration and Ventilation can have a major effect on aerosol concentration [22,23].
Filtration and reducing indoors aerosol concentrations
Filtration can be used as an effective adjunct to ventilation to reduce the concentration of indoor aerosols [4,25]. It is very important to consider the amount of clean air delivery in proportion to the volume of the room and the ventilation existing when using filtration technologies. There is a need to study the best way to improve filtration efficiency in ventilation, air conditioning, and heating systems [25]. Also, ventilation systems of airplanes designed to the reduction of the airborne virus’s build-up in passenger cabins can limit airborne transmission in the longer range. Therefore, the risk of further exposure in airplanes is due to face-to-face conversations and other close interactions.
The challenge of public health practices communicating appropriately when the disease is evolving rapidly is critical to disease control. The need to better understand the quantitative relationship between coronavirus dose and response to infectious disease and the importance of increasing testing to limit transmission is crucial. Cultural sensitivity is needed to convey information about modifying the behaviors that can put individuals at greater risk, for example, given family communities the importance of emotional health. It is very important to note that in some cases, due to weak urban or economic reasons, communities may not be able to implement filtration measures and improve building ventilation and even open windows that have been closed for security reasons. Mass transportation can also pose special challenges for those who are dependent on public transportation due to the problems of social distancing. There is a need to layer protective measures to the reduction of the virus spread, such as maximizing ventilation, masks, face shields if necessary, physical distancing, and hand hygiene. These combined actions are very important and should be promoted as the single management strategy. The value of these layered approaches in the interiors, including public transportation, schools, health care centers, office buildings, dental offices, and air travel, is enormous. Thus, aerosol particles protective approaches are important in environments where procedures may produce more aerosols (for example, dental offices and or during specific medical procedures). The risk of COVID-19 exposure in flight situations with high cabin air conditioning rates, up to 30/h air changes, is a key challenge in addressing travel-related behaviors that can increase the exposure risk, for example crowding when getting off the plane. Also, the mitigating approaches unintended consequences, including impacts on the energy consumption (such as ventilation systems), mental health, the economy, education, and the environment, are significant. Further research is needed to better understand SARS-CoV-2 transmission, but it should be emphasized that such studies do not eliminate need for immediately steps to prevent the further airborne routes infections. Public health professionals can take it immediately because they are trying to reduce risk of public exposure to COVID-19. Therefore, given the evidence of airborne aerosol particles transmission as the main potential pathway, it is a prudent strategy to take steps to limit this pathway especially indoors.
Declaration of interest
Funding: This review received no external funding and was initiated and funded by Dr. Ehsanifar Research Lab, Tehran, Iran.
Acknowledgments: We thank Dr. Ehsanifar Lab. Tehran, Iran.
- Dong Y, Mo X, Hu Y, Qi X, Jiang F, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020; 145: e20200702. PubMed: https://pubmed.ncbi.nlm.nih.gov/32179660/
- Guerra, F.M, Bolotin S, Lim G, Heffernan J, Deeks SL, et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017; 17: e420-e428. PubMed: https://pubmed.ncbi.nlm.nih.gov/28757186/
- Hamner L, Dubbel P, Capron I, Ross A, Jordan A, et al. High SARS-CoV-2 attack rate following exposure at a choir practice—Skagit County, Washington, March 2020. Morbidity and Mortality Weekly Report. 2020; 69: 6006-610.
- Ehsanifar M. Airborne aerosols particles and COVID-19 transition. Environ Res. 2021; 200: 111752. PubMed: https://pubmed.ncbi.nlm.nih.gov/34302822/
- Fisher KA, Tenforde MW, Feldstein LR, Lindsell CJ, Shapiro NI, et al. Community and close contact exposures associated with COVID-19 among symptomatic adults ≥ 18 years in 11 outpatient health care facilities—United States, July 2020. Morbidity and Mortality Weekly Report. 2020; 69: 1258-1264. PubMed: https://pubmed.ncbi.nlm.nih.gov/32915165/
- Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020; 395: 1907-1918. PubMed: https://pubmed.ncbi.nlm.nih.gov/32473681/
- Ehsanifar M, Rafati M, Wang J. Neurological complications related to COVID-19 infections following exposure to airborne aerosol particles. Clin Res Clin Trials. 2022; 5.
- Inglesby TV. Public health measures and the reproduction number of SARS-CoV-2. JAMA. 2020; 323: 2186-2187. PubMed: https://pubmed.ncbi.nlm.nih.gov/32356869/
- Imai N. Report 3: Transmissibility of 2019-nCoV, WHO Collaborating Centre for Infectious Disease Modelling. MRC Centre for Global Infectious Disease Analysis, J-IDEA, Imperial College London, UK, 2020.
- Alford RH, Kasel JA, Gerone PJ, Knight V. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med. 1966; 122: 800-804. PubMed: https://pubmed.ncbi.nlm.nih.gov/5918954/
- Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, et al. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. M Bio. 2016; 7: e00417-00416. PubMed: https://pubmed.ncbi.nlm.nih.gov/27094330/
- Lakdawala SS, Menachery VD. The search for a COVID-19 animal model. Science. 2020; 368: 942-943. PubMed: https://pubmed.ncbi.nlm.nih.gov/32467379/
- Halfmann PJ, Hatta M, Chiba S, Maemura T, Shufang Fan et al. Transmission of SARS-CoV-2 in domestic cats. New Engl J Med. 2020; 383: 592-594. PubMed: https://pubmed.ncbi.nlm.nih.gov/32402157/
- Richard M, Kok A, de Meulder D, Bestebroer TM, Lamers MM, Cet al. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nature Commun. 2020; 11: 1-6. PubMed: https://pubmed.ncbi.nlm.nih.gov/32641684/
- Ehsanifar M, Yavari Z, Motaghedifar MR, Rezaei M. Risk of activation of human viruses lurking in ambient following COVID-19 prevention supplies excessive use. J Community Med Health Solut. 2022; 3: 011-015. PubMed: https://www.communitymedjournal.com/articles/jcmhs-aid1015.php
- Yang YExuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. MedRxiv. 2020.
- Sia SF. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020; 583: 834-838.
- Coleman CM, Venkataraman T, Liu YV, Glenn GM, Smith GE, et al. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine. 2017; 35: 1586-1589. PubMed: https://pubmed.ncbi.nlm.nih.gov/28237499/
- Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol. 2015; 235: 185-195. PubMed: https://pubmed.ncbi.nlm.nih.gov/25270030/
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8: 420-422. PubMed: https://pubmed.ncbi.nlm.nih.gov/32085846/
- Zheng S, Fan J, Yu F, Feng B, Lou B, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020; 369: m1443. PubMed: https://pubmed.ncbi.nlm.nih.gov/32317267/
- Zhu S, Jenkins S, Addo K, Heidarinejad M, Romo SA, et al. Ventilation and laboratory confirmed acute respiratory infection (ARI) rates in college residence halls in College Park, Maryland. Environ Int. 2020; 137: 105537. PubMed: https://pubmed.ncbi.nlm.nih.gov/32028176/
- Wargocki P, Sundell J, Bischof W, Brundrett G, Fanger PO, et al. Ventilation and health in non-industrial indoor environments: report from a European multidisciplinary scientific consensus meeting (EUROVEN). Indoor Air. 2002; 12: 113-128. PubMed: https://pubmed.ncbi.nlm.nih.gov/12216467/
- Li Y, Qian H, Hang J, Chen X, Hong L, et al. Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. MedRxiv. 2020.
- Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, et al. Correlation between universal BCG vaccination policy and reduced mortality for COVID-19. MedRxiv. 2020.
- Liu L, Li Y, Nielsen PV, Wei J, Jensen RL. Short‐range airborne transmission of expiratory droplets between two people. Indoor Air. 2017; 27: 452-462. PubMed: https://pubmed.ncbi.nlm.nih.gov/27287598/
- Ehsanifar M. Does Exposure to Air Pollution Fine Particles and COVID-19 Contribute to the Risk of Ischemic Stroke? Health. 2021; 2: 1020.
- Jarvis CI, Zandvoort KV, Gimma A, Prem K, CMMID COVID-19 working group, et al. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020; 18: 1-10. PubMed: https://pubmed.ncbi.nlm.nih.gov/32375776/
- Cowling BJ, Ali ST, Ng TWY, Tsang TK, Li JCM, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020; 5: e279-e288. PubMed: https://pubmed.ncbi.nlm.nih.gov/32311320/
- Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ, et al. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013; 9: e1003205. PubMed: https://pubmed.ncbi.nlm.nih.gov/23505369/
- Leung NH, Chu DKW, Shiu EYC, Chan KH, McDevitt JJ, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature Med. 2020; 26: 676-680. PubMed: https://pubmed.ncbi.nlm.nih.gov/32371934/
- Xiao F, Sun J, Xu Y, Li F, Huang X, et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis. 2020; 26: 1920. PubMed: https://pubmed.ncbi.nlm.nih.gov/32421494/