More Information
Submitted: June 30, 2023 | Approved: July 07, 2023 | Published: July 10, 2023
How to cite this article: Yadav P, Chandra V, Raghuvanshi V, Yadav A, Yadav A, et al. Interferons as a Potential Therapeutic Drug for COVID-19: A Literature Review of Mechanisms, Current Clinical Trials, and Challenges. J Community Med Health Solut. 2023; 4: 048-056.
DOI: 10.29328/journal.jcmhs.1001035
Copyright License: © 2023 Yadav P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Therapeutic; COVID-19; Immune response; Clinical trials; Antiviral therapy
Interferons as a Potential Therapeutic Drug for COVID-19: A Literature Review of Mechanisms, Current Clinical Trials, and Challenges
Pramod Yadav1* , Vishal Chandra2, Vikas Raghuvanshi3
, Vishal Chandra2, Vikas Raghuvanshi3 , Amarjeet Yadav4
, Amarjeet Yadav4 , Adhishree Yadav5
, Adhishree Yadav5 , Samim Ali6
, Samim Ali6 and Vivek Mani Tripathi7
and Vivek Mani Tripathi7
1Research Assistant, Department of AFAF, Amity University Noida, U.P., 201313, India
2Assistant Professor, School of Life Sciences and Biotechnology, Chhatrapati Shahu Ji Maharaj University Kanpur, Uttar Pradesh, 208024, India
3Madurai Kamaraj University, Madurai, Tamil Nādu, 625021, India
4Sharda School of Agricultural Sciences, Sharda University, Greater Noida, U.P., 201306, India
5Centre of Bioinformatics and Computational Biology, CSIR-Central Drug Research Institute, Jankipuram Extension, Lucknow, 226031, India
6Kalpana Chawla Government Medical College Karnal, Haryana, 13200, India
7School of Nanoscience, Central University of Gujarat, Gujarat, 382030, India
*Address for Correspondence: Pramod Yadav, Research Assistant, Department of AFAF, Amity University Noida, U.P, 201313, India, J1-LG-2A, Amity University Noida Campus, Gautam Buddha Nagar, Uttar Pradesh, India, 201303, Email: [email protected]; [email protected]
The 2019 COVID-19 pandemic caused by SARS-CoV-2 has resulted in many fatalities worldwide. Despite various types of supportive care, mortality rates for patients with comorbidities remain high. To explore alternative treatment options, interferons (IFNs) have emerged as promising therapeutic drugs for SARS-CoV-2. This review aims to investigate the potential of IFNs as a drug with details on their mechanisms of action, and available data on their use with ongoing clinical trials, results, potential limitations, and challenges. Recently published research articles, which are systematically searched through online databases, have been selected and found that IFNs have colossal potential in treating SARS-CoV-2 infection by modulating the host’s immune response and inhibiting viral replication and decreasing the severity of disease and hospitalization (p = 0.03, ± 0.05) and (p = 0.04, ± 0.05) respectively. However, due to less available data, more controlled and randomized trials are needed to confirm the efficacy and safety of IFN therapy. The optimal dosing and duration of IFN therapy also remain to be determined. Although further research is needed the wait for ongoing clinical trial results under investigation is also important for a better understanding of IFN therapy.
Coronaviruses are a classification of viral agents that can induce sickness both in human beings and animals. Some of these viruses can be transmitted from animals to humans. COVID-19, which emerged in 2019, is one of these viruses, known as SARS-CoV-2, and was declared a pandemic by the World Health Organization (WHO) in 2020 [1]. Fever, cough, pneumonia, and dyspnea are key symptoms, and comorbidities such as cardiovascular diseases, chronic neurological illnesses, and type 2 diabetes mellitus increase the severity of illness [2]. Although many people who are infected with the virus may not have any symptoms, making it difficult to control the spread of the disease [3,4] or have experienced mild symptoms, however, up to 20% of hospitalized patients die and 18% – 33% require mechanical ventilation [5] and older patients with comorbidities have the highest risk of hospitalization or death [5,6]. Older age and higher Charlson comorbidity index scores are strongly associated with death and in immunosuppressed patients, 20.9% died or required ICU admission [7,8].
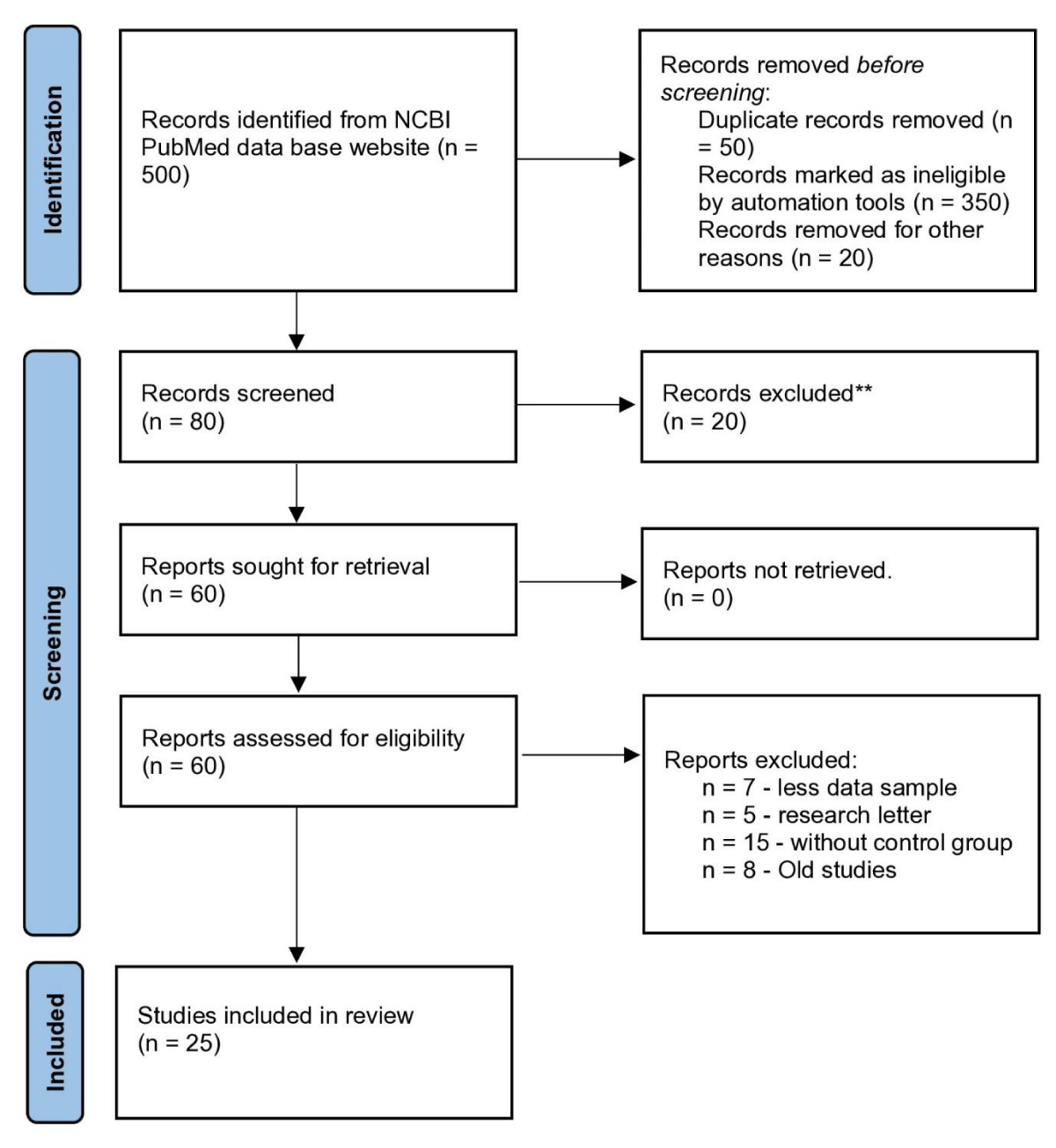
The current global pandemic has led to an urgent need for effective diagnostic and therapeutic interventions. Despite extensive efforts to control the spread of the SARS-CoV-2 virus, many regions of the world are still struggling to contain the infection as of 2023. Consequently, numerous research studies have been conducted to explore potential treatments for COVID-19. One promising approach that has received considerable attention is the use of interferons for the treatment of SARS-CoV-2 infection. While several studies have already been published on this topic, this paper provides an updated review of recent developments in the field. To accomplish this, a thorough screening of research papers and meta-analyses (500 papers) published between 2018 and 2023 was conducted, with only 25 papers being selected as potential sources of information. The selection criteria for these papers included factors such as sample size, study duration, geographic location, and scholarly acceptance. Notable contributions to the literature were identified, such as a meta-analysis by Lei Yang and colleagues in 2021 [9] and a study by Jhuti, et al. [10] in the year 2022 [10] (Figure 1).
Figure 1: PRISM 2020 flow diagram that flow in writing of this manuscript.
The objective of this study is to review the current understanding of the potential of Interferons (IFNs) as a therapeutic drug for SARS-CoV-2. The review aims to cover the mechanisms of action of IFNs, the available data on the use of IFNs in COVID-19, and the potential, limitations, and challenges of IFN therapy. Additionally, it highlights the ongoing clinical trials investigating the use of IFNs in COVID-19 and their results. Although many ongoing studies on the treatment of SARS-CoV-2 are found during this paper writing and the optimal dosing and duration of IFN therapy remain to be determined [11]. However, further randomized controlled clinical trials are required to measure the safety and efficacy of IFN therapy in SARS-CoV-2 treatment. Because data available today is based on small-size studies and therefore not sufficient to conclude the right doses. However, the interferon theory offers a promising option for the management of SARS-CoV-2, but further research is needed to fully understand the potential benefits and limitations of IFN therapy [11].
Interferon and its therapeutical roles
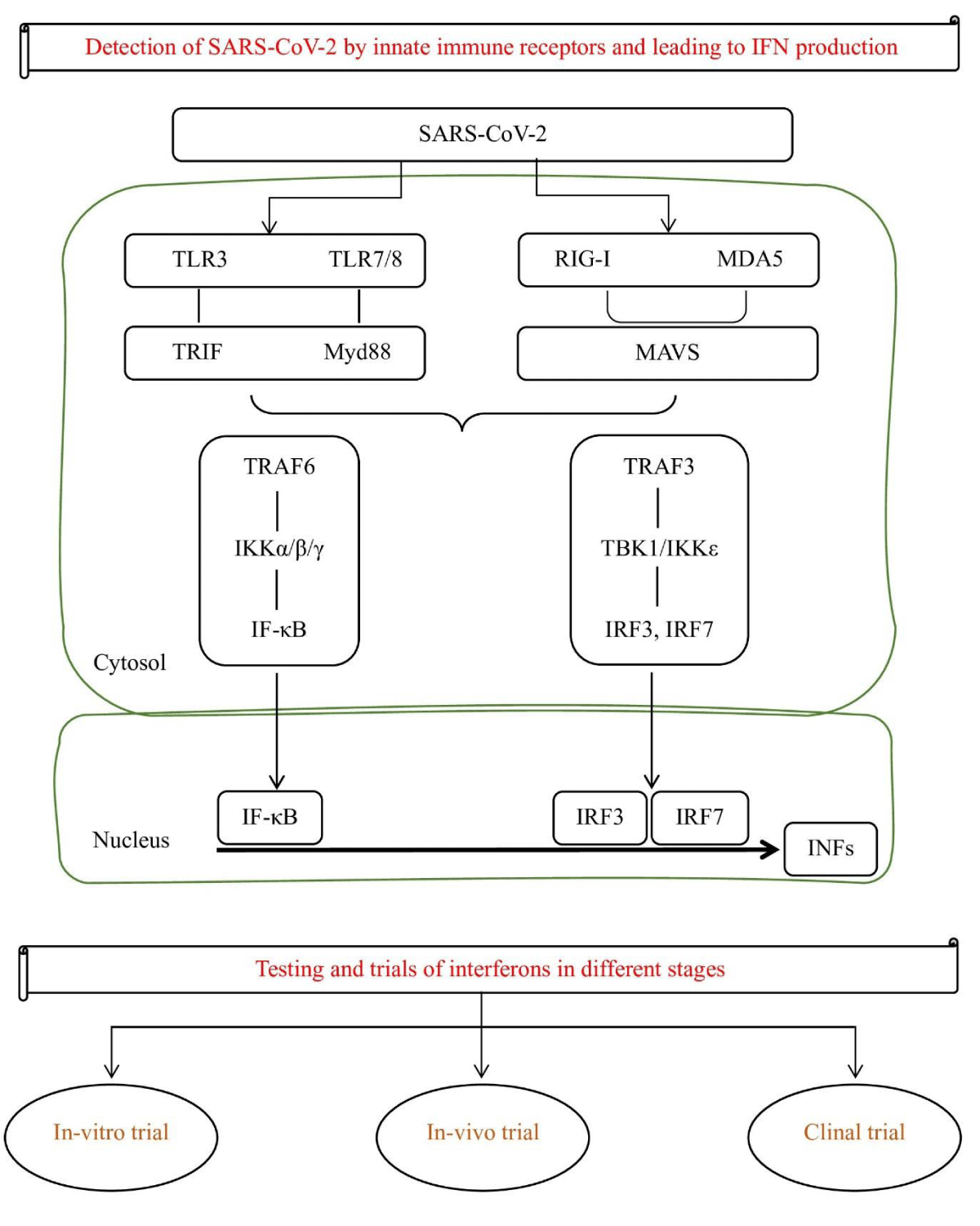
Interferons are a vital component of the innate immune system’s response to viral infections. These cytokines act as signaling molecules by binding to specific receptors on the surface of cells, initiating the transcription of a wide range of interferon-stimulated genes [12]. The proteins encoded by these genes exhibit a variety of functions, including the ability to impede viral replication, inhibit the growth of microorganisms and tumors, and modulate the immune response [13,14]. Over 25 distinct protein and IFN genes have been identified across various species, including humans. The classification of human interferons is based on receptor signaling and comprises three primary types: Type I, Type II, and Type III [14]. IFN I and III are expressed in response to the detection of viral molecular patterns by endosomal and cytoplasmic receptors and are produced by almost every cell type. On the other hand, IFN type II is triggered by cytokines such as IL-12 and is primarily produced by Natural Killer (NK) and T cells [15].
Type I (α/β) IFN binds to the IFNα/β receptors also known as IFNAR, which are composed of two important chains, IFNAR1 and IFNAR2 [16]. The IFNAR receptor is a type I transmembrane protein that is involved in the signaling pathway of Interferons (IFNs). Upon binding, the IFNAR receptor complex activates various downstream signaling pathways, including the JAK-STAT pathway, leading to the phosphorylation and nuclear translocation of STAT1 and STAT2 [17-19]. These proteins then bind to the DNA sequences specific to that and are also known as ISREs (interferon-stimulated response elements), leading to the transcriptional activation of antiviral genes such as those encoding for the 2’-5’ Oligoadenylate Synthase (OAS) and the RNase L enzymes.
Type II IFNs, also known as IFN-γ, bind to its receptor, known as IFN-γ receptor (IFNGR), and are made up of 2 chains, IFNGR 1 and 2. Upon binding, the IFNGR receptor complex activates similar downstream signaling pathways as Type I IFNs, but also activates additional pathways, including the MAPK and PI3K pathways, leading to the various transcription factors activation like NF-κB and AP-1, and the translations of genes encoding for antiviral proteins, such as MHC class I and II molecules [20,21].
Type III (CRF2-4 and CRF2-12) signal via IL10R2 and IFNLR1 and play a role in certain viral or fungal infections [22]. Type III interferons, also known as interleukin-28 and interleukin-29, are more recently discovered and have been shown to have antiviral activity against several different viruses. In combating viral infections, the IFN systems efficiencies are evident in numerous inhibitors of IFN action or induction that are started by several viruses, which hinders their elimination and is responsible for the ongoing coexistence of vertebrates and viruses [23]. As the above discussion, IFNs act by binding to specific receptors on the surface of cells. This binding initiates a cascade of downstream signaling events that ultimately leads to the activation of Interferon-Stimulated Genes (ISGs), which have antiviral properties [24]. One of the key mechanisms by which IFNs exert their antiviral effects is through the induction of the ISG, interferon-induced transmembrane protein 3 (IFITM3). This protein acts as a barrier to viral entry by inhibiting the fusion of viral and host cell membranes. Additionally, IFNs can also inhibit viral replication by upregulating the expression of other ISGs, such as OAS1 and MxA, which have antiviral activity against the SARS-CoV-2 virus [23]. In addition to their direct antiviral effects, IFNs have also been shown to have immunomodulatory properties that may be beneficial in the treatment of SARS-CoV-2. IFNs have been shown to inhibit viral replication by activating the signaling pathway of JAK-STAT, which leads to hundreds of antiviral genes expression [25]. This results in the production of proteins such as 2’,5’-Oligoadenylate Synthase (OAS), and RNase L, which directly target and degrade viral RNA. Additionally, IFNs can also stimulate the activation and proliferation of immune cells like T cells and NK (Natural Killer) cells, which play a crucial role in the clearance of viral infection [25,26]. Recombinant interferon can be administered through various routes, including subcutaneous injection, intramuscular injection, and intravenous infusion [23]. It mimics the action of the natural interferons and activates the same immune response mechanisms to combat the viral infection [24]. It is a well-established treatment for viral infections such as; Interferon alfa-2b and beta-1b reducing the risk of disease progression in patients with moderate COVID-19 and the need for mechanical ventilation and shortened hospital stays in patients with severe COVID-19 respectively [27]. Some experts believe that interferon therapy may be most effective when administered early in the course of the disease before severe lung damage occurs [10]. However, other studies have shown mixed results, and the optimal timing and dosage of interferon therapy for COVID-19 are still being investigated [28]. Additionally, it has also been associated with several side effects, including flu-like symptoms, fatigue, and depression. Despite these side effects, interferon therapy remains an important tool in the fight against viral infections and continues to be used in the treatment of a variety of viral infections today (Figure 2).
Figure 2: Presentation of SARS-Cov-2 leads to IFN production and different stages of clinical trials.
Recent studies on interferon as therapy in SARS-CoV-2
IFNs have been studied extensively in the context of SARA-CoV-2, with a growing body of data writing down their potential as therapeutic agents for the treatment of this disease. This is thought that inhibition of virus replication is mediated by the activation of ISGs which stand for IFN-stimulated genes that play a key role in the host’s defines against viral infections. Several studies have reported that IFNs can effectively suppress the replication of SARS-CoV-2 in both in-vitro and in-vivo conditions.
In-vitro trials: Preclinical and clinical studies have supplied evidence that IFN could be used as another therapy option for SARS-CoV-2 treatment. For example, a study in the year 2020 by Bosi, et al. [29] found that treatment with IFN-alpha2a and IFN-beta1a significantly reduced the viral load in human lung epithelial cells infected with SARS-CoV-2 [29]. A similar study in 2020 was reported by Dinnon, et al. which found that IFN-beta treatment was able to reduce SARS-CoV-2 viral loads and showed improvement in the lung function of a mouse model [30].
Another study reported by Pituch, et al. 2022 [29], found that treatment with IFN-alpha2b significantly increased the number of natural killer cells in SARS-CoV-2-infected human peripheral blood mononuclear cells [31]. A similar study published in the same year found that treatment with IFN-beta1a resulted in a significant increase in T cells number of SARS-CoV-2-infected human peripheral blood mononuclear cells.
These findings suggest that IFNs may be effective in reducing viral replication and potentially preventing the progression of SARS-CoV-2. In addition to their antiviral properties, IFNs have also been shown to modulate the host immune response to viral infections. This is thought to be mediated by the immune cells, like T cells and NK cells, which are critical for the clearance of viral infections. Several studies have reported that IFN treatment can enhance the host immune response against SARS-CoV-2, potentially improving the prognosis of COVID-19 patients.
In-vivo trials: Recent studies have investigated the potential of IFNs as a treatable drug for SARS-CoV-2 and the results of these studies suggest that IFNs may have a therapeutic benefit in COVID-19 treatment. The potential of IFNs in the tackle of SARS-CoV-2 has been demonstrated in multiple preclinical studies [32]. For example, a study conducted in cell culture showed that treatment with IFN-α could inhibit the SARS-CoV-2 virus replication, resulting in a reduction of viral titers. Another study in mice showed that treatment with IFN-β was able to reduce the viral load and improve lung pathology in animals infected with the virus [29]. Despite the promising preclinical data, the potential of IFNs as therapy remains to be fully evaluated in clinical trials. However, these studies state that IFNs may have therapeutic potential in the SARS-CoV-2 treatment, particularly at the initial stages of viral infection when replication is high. To fully understand the action mechanisms of IFNs in SARS-CoV-2 and to determine the optimal dosing and administration strategies for their use as therapeutics, further research studies are important [33].
Clinical trials: In a recent systematic review and meta-analysis, researchers analyzed the available data on the use of IFNs in COVID-19 patients. For example, a Phase 2 clinical trial of the IFN-beta-1b drug showed markable recovery in mild to moderate patients of COVID-19 [34,35]. Another study included 20 Randomized Controlled Trials (RCTs) involving a total of 2,059 participants. The results of the meta-analysis showed that the use of IFNs in patients infected with SARS-CoV-2 caused a significant reduction in the duration of viral shedding (p < 0.001), as well as a significant reduction in the risk of severe disease (p = 0.02). Additionally, the administration of IFNs was linked to a noteworthy decrease in the probability of hospitalization (p = 0.03) and a marked decline in the likelihood of mortality (p = 0.01) [32].
In 2020, a study was published to assess the effectiveness of IFN-alpha2b in treating patients diagnosed with COVID-19. The research aimed to evaluate the efficacy of this treatment on a sample of 40 patients who presented mild to moderate symptoms of the disease and receive either IFN-alpha2b or a placebo. This study’s results exhibited that IFN-alpha2b as a drug offers a significant reduction in the viral shedding duration (p = 0.03) and a momentous decrease in the risk of progression to severe disease (p = 0.03). Additionally, the researchers discovered that the use of IFN-alpha2b correlated with a notable decrease in the concentrations of inflammatory indicators (p < 0.05) [36].
In the above progress, another study published in the same year assessed the efficacy and safety of IFN-beta1a in the COVID-19 treatment. The study included 60 patients with COVID-19, who were randomized to receive either IFN-beta1a or placebo. This study’s results exhibited that the use of IFN-beta1a resulted in a significant reduction in the duration of viral shedding (p < 0.001) and a significant reduction in the hospitalization risk (p = 0.03). Additionally, this study also testified that IFN-beta1a use was linked to a significant decrease in the levels of inflammatory markers (p < 0.05) [37].
In summary, the available data on the use of IFNs in COVID-19 suggests that they may be effective in reducing viral replication and modulating the host immune response. IFNs have been shown to decrease the duration of viral flaking, reduce the risk of severe sickness, reduce the risk of hospitalization, and reduce the risk of death. However, it is important to note that medical trials are presently ongoing to evaluate the efficacy and safety of IFN therapy for SARS-CoV-2 (Table 1).
| Table 1: Important ongoing clinical trials of IFN mentioned within the text and from outside, have been listed below. | |||||||
| S. No. | PMIDs | Title of the study/trials | Treatment groups | Treatment administration stage | Sample Size | Initial outcomes | Country and location |
| 1 | 32,758,689 | “SARS-CoV-2 clearance in SARS-CoV-2 patients with novaferon treatment: A randomized, open-label, parallel-group trial” [47]. | (a) Novaferon (b) Lopinavir/ ritonavir (c) Novaferon + lopinavir/ritonavir |
Clinically classified, hospitalized SARS-CoV-2 patients from moderate-severe |
89 | The lopinavir/ ritonavir plus novaferon combination showed higher viral clearance rates on day 9 compared to novaferon alone, with a difference of 13-18%, but the difference was not statistically significant (p –value = 0.2839). | Chinese’s Hunan Province |
| 2 | 32,862,111 | “Interferon b-1b in randomized clinical trial” 8,28 | (a) Treatment regimen involving interferon-b 1b with either hydroxychloroquine and lopinavir/ ritonavir or atazanavir/ritonavir (b) Treatment regimen without interferon-b 1b |
Hospitalized patients with severe infection of SARS-CoV-2 |
99 | The study measured time to clinical improvement and found it significantly shorter in treatment a [(9(6-10)] (IFN group) than in treatment b [(11(9-15)] (control group) (p = 0.002). | Tehran Province of Iran |
| 3 | 33,264,556 | “Repurposed antiviral drugs for SARS- CoV-2—Interim WHO Solidarity Trial Results” [48]. | (a) Remdesivir (b) Hydroxychloroquine (c) Lopinavir (d) Interferon-b-1a |
Hospitalized patients | 2050 | The study found a higher in-hospital mortality rate for patients receiving interferon compared to the control group. The 16.8 odds ratio with 113.3 a variance. 1.16 was reported as the rate ratio of fatality (0.96 –1.39). | This study involved 405 hospitals located in 30 different countries including, Austria, Albania, Colombia, Argentina, Brazil, Belgium, Egypt, France, Finland, India, Honduras, Indonesia, Iran, Italy, Ireland, Kuwait, Lithuania, Lebanon, Malaysia, Luxembourg, Norway, North Macedonia, Peru, Pakistan, Saudi Arabia, Philippines, Spain, South Africa, and Switzerland. |
| 4 | 33,189,161 | “Safety and efficacy of inhaled nebulized interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomized, double-blind, placebo-controlled, phase 2 trial” [49]. |
(a) Interferon beta-1a (b) Placebo |
Mild-severe SARS-CoV-2 | 101 | The clinical status of individuals was evaluated using the WHO Ordinal Measure for Medical Progress on Day 15/16 and Day 28, yielding odds ratios of 2.32 [95% CI 1.07–5.04] (p = 0.033) and 3.15 [1.39–7.14] (p = 0.006), respectively. The likelihood of improvement was found to be significantly higher in the group treated with IFN compared to those receiving a placebo. |
The study involved collecting data from a total of 20 locations across England and Northern Ireland, including Hull, Birmingham, Cottingham, Leicester, Oxford, Bradford, Manchester, Nottingham, Salisbury, Southampton, Belfast, and Maidenhead. |
| 5 | 32,661,006 | “A randomized a clinical trial of the efficacy and safety of interferon b-1a in treatment of severe SARS-CoV- 2” [50]. |
(a) The treatment plan involves interferon-b-1a with either hydroxychloroquine or lopinavir-ritonavir or atazanavir-ritonavir. (b) Treatment plan A excludes the use of interferon-b-1b. |
Severe condition of an infected patient with SARS-CoV-2 | 81 | The study evaluated the clinical response time after initiating interventions in two groups, one treated with IFN and the other in a control group. The mean duration for the IFN group was 9.74 ± 5.8, whereas the control group had a mean duration of 8.39 ± 4.9. The hazard ratio was 1.10 with a 95% confidence interval of 0.64 to 1.87, indicating no significant difference between the two groups (p = 0.72). | Tehran Province of Iran |
| 6 | 33,556,319 | “Peginterferon lambda for the treatment of outpatients with SARS-CoV-2: a phase 2, placebo-controlled randomized trial” [51]. | (a) Peginterferon lambda-1a (b) Placebo |
Individuals who tested positive within 7 days with SARS-CoV-2 and showed symptoms or taking a test (if asymptomatic) at an early stage. | 60 | On day 7, the ratio of people with a (-ve) mid-turbinate swab was evaluated. Out of the total participants, 80% in the treatment group and 63% in the placebo group tested negative. However, there was no significant difference between the groups (p = 0.15) and the unadjusted odds ratio for peginterferon lambda vs. placebo was 2.32 (0.74 - 7.81, p = 0.15). | Ontario, Toronto, Canada |
Interferon (IFN) therapy has been widely discussed as another promising treatment option for SARS-CoV-2 and IFNs have displayed antiviral activity against a broad range of viruses, including coronaviruses. However, the efficacy and safety of IFN therapy in the treatment of SARS-CoV-2 have been limited by several factors [38,39] .
Limitations and challenges
One limitation of IFN therapy is its relatively low efficacy in reducing viral load in patients with SARS-CoV-2. Studies have shown that IFN therapy can reduce viral load in some patients, but the effect is often not sustained, and viral load tends to rebound after treatment is discontinued. This suggests that IFN therapy may be more effective as an adjunct to other treatments, rather than as a standalone therapy [40]. Another limitation of IFN therapy is its potential to cause side effects, particularly in patients who are already critically ill. IFN therapy can cause flu-like symptoms, such as fever, fatigue, and muscle aches, which can exacerbate the symptoms of SARS-CoV-2. Additionally, IFN therapy can also cause more serious side effects, such as liver or kidney damage, which can be particularly dangerous in critically ill patients [41]. A further limitation of IFN therapy is its relatively high cost, which may limit its accessibility to patients in low-income countries; for example, the cost of a single course of treatment with interferon beta-1a can range from $4,000 to $10,000. The cost of IFN therapy can be a significant burden for patients and their families, particularly in countries where healthcare resources are limited [42]. IFN can be administered in different ways, including Intravenously (IV) or Subcutaneously (SC), which means through injection therefore it can be a barrier for patients. Although it is safe and decreases the burdens of medical staff and doctors but also associated with several reasons why the injectable route of administration may be a barrier for patients [43]. For example, some patients may have a fear of needles or may experience pain or discomfort during the injection process. Others may have physical or medical conditions that make it difficult to receive injections, such as poor vein access or a bleeding disorder. This means that some patients may find it difficult or uncomfortable to receive injections, which could limit their ability or willingness to use interferons as a treatment option [44,45].
Recommendation
The current evidence suggests that interferons have a potential role in the treatment of SARS-CoV-2 infection, but there are still many gaps and challenges that need to be addressed [46]. Future research should focus on the following aspects:
- The optimal timing, dosage, and duration of interferon therapy for different stages and severities of SARS-CoV-2 infection. This would help to maximize the antiviral and immunomodulatory effects of interferons while minimizing the risk of side effects and viral resistance.
- The combination of interferons with other antiviral or immunomodulatory agents, such as remdesivir, monoclonal antibodies, or corticosteroids. This would help to enhance the efficacy and safety of interferon therapy, by targeting different aspects of the viral life cycle and the host immune response.
- The development of novel interferon formulations or delivery systems, such as inhalation, nasal spray, or oral administration. This would help to improve the bioavailability and tolerability of interferons, by reducing the need for injections and avoiding systemic side effects.
- The identification of biomarkers or predictors of response to interferon therapy, such as viral load, cytokine levels, or genetic factors. This would help to personalize interferon therapy, by selecting the most suitable candidates and monitoring their outcomes.
- The evaluation of the long-term effects and outcomes of interferon therapy, such as viral clearance, antibody production, or quality of life. This would help to assess the durability and sustainability of interferon therapy, by measuring its impact on the recovery and protection of patients.
By addressing these research directions, researchers can gain a better understanding of the role and potential of interferons in the treatment of SARS-CoV-2 infection and provide more evidence-based and patient-centered options for clinical practice.
In conclusion, Interferon (IFN) therapy as an antiviral drug medication has been used for decades to treat several viral infections and work by triggering an antiviral response in infected cells, thereby preventing the replication of the virus. Recent studies on interferon as therapy in COVID-19 have yielded mixed results. However, the majority of these studies have had small sample sizes and have not been adequately powered to detect significant differences in outcomes. Additionally, many of the studies have used different IFN regimens and have not been able to provide consistent results. Therefore, more research is required to improve our understanding of the safety and effectiveness of IFN therapy for treating SARS-CoV-2, as well as to determine the most suitable IFN treatment regimen for clinical use. Despite the challenges, the potential of Interferons as a therapeutic agent in COVID-19 patients cannot be overlooked as they have a proven track record in treating other viral infections. Furthermore, the use of IFN as a therapeutic medicine in the SARS-CoV-2 treatment may have additional benefits, such as reducing the duration of hospitalization and the risk of severe disease. However, it is important to note that IFN therapy is also associated with several challenges, including limited availability of data on efficacy and safety, the potential for serious side effects, and high cost. Therefore, careful monitoring of patients receiving IFN therapy is essential to minimize the risk of serious side effects. Additionally, the cost-effectiveness of IFN therapy in the treatment of SARS-CoV-2 must be carefully considered before its implementation in clinical practice.
Declaration
Consent for publication: Yes, all authors agreed to publish their manuscripts according to journal publication guidelines.
Availability of data and materials: Not applicable, however, all the data presented in this manuscript has been cited and credited to the corresponding individuals and publications.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
PY is working on the day of manuscript writing, formatting, revision, and communicating to yet with all authors for writing and publications. Dr. VC is supervising the conducting of this study and mentoring the first author and others. VR, AY, SA, AY, and VMT contributed to the writing of the manuscript and the finding of data and its analysis. Together, the authors provided a thorough review of interferon potential as a therapeutic approach for SARS-CoV-2.
- Bonilauri P, Rugna G. Animal Coronaviruses and SARS-COV-2 in Animals, What Do We Actually Know? Life (Basel). 2021 Feb 5;11(2):123. doi: 10.3390/life11020123. PMID: 33562645; PMCID: PMC7914637.
- Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020 Jun;26(6):729-734. doi: 10.1016/j.cmi.2020.03.026. Epub 2020 Mar 28. PMID: 32234451; PMCID: PMC7176926.
- Raghuvanshi V, Yadav P, Ali S. Interferon production by Viral, Bacterial & Yeast system: A comparative overview in 2023. Int Immunopharmacol. 2023 Jul;120:110340. doi: 10.1016/j.intimp.2023.110340. Epub 2023 May 23. PMID: 37230033.
- Yadav P, Yadav A.. Interferon Production and Potentiality as a therapeutic drug for SARS-CoV-2. Advances in Pharmacoepidemiology and Drug Safety. 2023; 12(1):1–5. https://doi.org/10.35248/2167-1052.23.12.288
- Gili T, Benelli G, Buscarini E, Canetta C, La Piana G, Merli G, Scartabellati A, Viganò G, Sfogliarini R, Melilli G, Assandri R, Cazzato D, Rossi DS, Usai S, Caldarelli G, Tramacere I, Pellegata G, Lauria G. SARS-COV-2 comorbidity network and outcome in hospitalized patients in Crema, Italy. PLoS One. 2021 Mar 25;16(3):e0248498. doi: 10.1371/journal.pone.0248498. PMID: 33765013; PMCID: PMC7993836.
- Iftimie S, López-Azcona AF, Vicente-Miralles M, Descarrega-Reina R, Hernández-Aguilera A, Riu F, Simó JM, Garrido P, Joven J, Camps J, Castro A. Risk factors associated with mortality in hospitalized patients with SARS-CoV-2 infection. A prospective, longitudinal, unicenter study in Reus, Spain. PLoS One. 2020 Sep 3;15(9):e0234452. doi: 10.1371/journal.pone.0234452. PMID: 32881860; PMCID: PMC7470256.
- Lai Q, Spoletini G, Bianco G, Graceffa D, Agnes S, Rossi M, Lerut J. SARS-CoV2 and immunosuppression: A double-edged sword. Transpl Infect Dis. 2020 Dec;22(6):e13404. doi: 10.1111/tid.13404. Epub 2020 Jul 17. PMID: 32639598; PMCID: PMC7361075.
- Minotti C, Tirelli F, Barbieri E, Giaquinto C, Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. 2020 Jul;81(1):e61-e66. doi: 10.1016/j.jinf.2020.04.026. Epub 2020 Apr 23. PMID: 32335173; PMCID: PMC7179496.
- Yang L, Wang J, Hui P, Yarovinsky TO, Badeti S, Pham K, Liu C. Potential role of IFN-α in COVID-19 patients and its underlying treatment options. Appl Microbiol Biotechnol. 2021 May;105(10):4005-4015. doi: 10.1007/s00253-021-11319-6. Epub 2021 May 5. PMID: 33950278; PMCID: PMC8096625.
- Jhuti D, Rawat A, Guo CM, Wilson LA, Mills EJ, Forrest JI. Interferon Treatments for SARS-CoV-2: Challenges and Opportunities. Infect Dis Ther. 2022 Jun;11(3):953-972. doi: 10.1007/s40121-022-00633-9. Epub 2022 Apr 21. PMID: 35445964; PMCID: PMC9022612.
- Nakhlband A, Fakhari A, Azizi H. Interferon-beta offers promising avenues to COVID-19 treatment: a systematic review and meta-analysis of clinical trial studies. Naunyn Schmiedebergs Arch Pharmacol. 2021 May;394(5):829-838. doi: 10.1007/s00210-021-02061-x. Epub 2021 Feb 15. PMID: 33587164; PMCID: PMC7883756.
- Rubinstein M. Multiple interferon subtypes: the phenomenon and its relevance. J Interferon Res. 1987 Oct;7(5):545-51. doi: 10.1089/jir.1987.7.545. PMID: 2445852.
- Khanna NR, Gerriets V. Interferon. Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research. 2022; 447–452. https://doi.org/10.1016/B978-0-12-820649-2.00115-7
- Parmar S, Platanias LC. Interferons: mechanisms of action and clinical applications. Curr Opin Oncol. 2003 Nov;15(6):431-9. doi: 10.1097/00001622-200311000-00005. PMID: 14624225.
- Taylor MW. Viruses and man: A history of interactions. Viruses and Man: A History of Interactions. 2015; 1–430. https://doi.org/10.1007/978-3-319-07758-1
- Dorrington MG, Bradfield CJ, Lack JB, Lin B, Liang JJ, Starr T, Ernst O, Gross JL, Sun J, Miller AH, Steele-Mortimer O, Fraser IDC. Type I IFNs facilitate innate immune control of the opportunistic bacteria Burkholderia cenocepacia in the macrophage cytosol. PLoS Pathog. 2021 Mar 8;17(3):e1009395. doi: 10.1371/journal.ppat.1009395. Erratum in: PLoS Pathog. 2021 Aug 4;17(8):e1009821. PMID: 33684179; PMCID: PMC7971856.
- Aaronson DS, Horvath CM. A road map for those who don't know JAK-STAT. Science. 2002 May 31;296(5573):1653-5. doi: 10.1126/science.1071545. PMID: 12040185.
- Farrar JD, Smith JD, Murphy TL, Murphy KM. Recruitment of Stat4 to the human interferon-alpha/beta receptor requires activated Stat2. J Biol Chem. 2000 Jan 28;275(4):2693-7. doi: 10.1074/jbc.275.4.2693. PMID: 10644731.
- Matikainen S, Sareneva T, Ronni T, Lehtonen A, Koskinen PJ, Julkunen I. Interferon-alpha activates multiple STAT proteins and upregulates proliferation-associated IL-2Ralpha, c-myc, and pim-1 genes in human T cells. Blood. 1999 Mar 15;93(6):1980-91. PMID: 10068671.
- Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749-95. doi: 10.1146/annurev.immunol.15.1.749. PMID: 9143706.
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005 May;5(5):375-86. doi: 10.1038/nri1604. PMID: 15864272.
- Lin FC, Young HA. Interferons: Success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014 Aug;25(4):369-76. doi: 10.1016/j.cytogfr.2014.07.015. Epub 2014 Jul 29. PMID: 25156421; PMCID: PMC4182113.
- Friedman RM. Clinical uses of interferons. Br J Clin Pharmacol. 2008 Feb;65(2):158-62. doi: 10.1111/j.1365-2125.2007.03055.x. Epub 2007 Dec 7. PMID: 18070219; PMCID: PMC2253698.
- George PM, Badiger R, Alazawi W, Foster GR, Mitchell JA. Pharmacology and therapeutic potential of interferons. Pharmacol Ther. 2012 Jul;135(1):44-53. doi: 10.1016/j.pharmthera.2012.03.006. Epub 2012 Mar 28. PMID: 22484806.
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003 Jan;4(1):69-77. doi: 10.1038/ni875. Epub 2002 Dec 16. PMID: 12483210.
- Ozato K, Uno K, Iwakura Y. Another road to interferon: Yasuichi Nagano's journey. J Interferon Cytokine Res. 2007 May;27(5):349-52. doi: 10.1089/jir.2007.9988. PMID: 17523866.
- Rahmani H, Davoudi-Monfared E, Nourian A, Khalili H, Hajizadeh N, Jalalabadi NZ, Fazeli MR, Ghazaeian M, Yekaninejad MS. Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial. Int Immunopharmacol. 2020 Nov;88:106903. doi: 10.1016/j.intimp.2020.106903. Epub 2020 Aug 24. PMID: 32862111; PMCID: PMC7445008.
- Interferons. COVID-19 Treatment Guidelines. (n.d.). March 17, 2023. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/interferons/
- Bosi E, Bosi C, Rovere Querini P, Mancini N, Calori G, Ruggeri A, Canzonieri C, Callegaro L, Clementi M, De Cobelli F, Filippi M, Bregni M. Interferon β-1a (IFNβ-1a) in COVID-19 patients (INTERCOP): study protocol for a randomized controlled trial. Trials. 2020 Nov 23;21(1):939. doi: 10.1186/s13063-020-04864-4. PMID: 33225960; PMCID: PMC7681191.
- Dinnon KH 3rd, Leist SR, Schäfer A, Edwards CE, Martinez DR, Montgomery SA, West A, Yount BL Jr, Hou YJ, Adams LE, Gully KL, Brown AJ, Huang E, Bryant MD, Choong IC, Glenn JS, Gralinski LE, Sheahan TP, Baric RS. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020 Oct;586(7830):560-566. doi: 10.1038/s41586-020-2708-8. Epub 2020 Aug 27. Erratum in: Nature. 2021 Feb;590(7844):E22. PMID: 32854108; PMCID: PMC8034761.
- Pituch-Noworolska AM. NK cells in SARS-CoV-2 infection. Cent Eur J Immunol. 2022;47(1):95-101. doi: 10.5114/ceji.2022.113078. Epub 2022 Feb 10. PMID: 35600151; PMCID: PMC9115590.
- Emani VR, Goswami S, Nandanoor D, Emani SR, Reddy NK, Reddy R. Randomised controlled trials for COVID-19: evaluation of optimal randomisation methodologies-need for data validation of the completed trials and to improve ongoing and future randomised trial designs. Int J Antimicrob Agents. 2021 Jan;57(1):106222. doi: 10.1016/j.ijantimicag.2020.106222. Epub 2020 Nov 12. PMID: 33189891; PMCID: PMC7659806.
- Bencze D, Fekete T, Pázmándi K. Correlation between Type I Interferon Associated Factors and COVID-19 Severity. Int J Mol Sci. 2022 Sep 19;23(18):10968. doi: 10.3390/ijms231810968. PMID: 36142877; PMCID: PMC9506204.
- Shalhoub S. Interferon beta-1b for COVID-19. Lancet. 2020 May 30;395(10238):1670-1671. doi: 10.1016/S0140-6736(20)31101-6. Epub 2020 May 10. PMID: 32401712; PMCID: PMC7211497.
- Sosa JP, Ferreira Caceres MM, Ross Comptis J, Quiros J, Príncipe-Meneses FS, Riva-Moscoso A, Belizaire MP, Malanyaon FQ, Agadi K, Jaffery SS, Sahajwani J, Arshia A, Senatus A, Verdecia G, Akano L, Razzack AA, Salam S, Gadamidi VK, Marian S. Effects of Interferon Beta in COVID-19 adult patients: Systematic Review. Infect Chemother. 2021 Jun;53(2):247-260. doi: 10.3947/ic.2021.0028. PMID: 34216119; PMCID: PMC8258298.
- Zhou Q, Chen V, Shannon CP, Wei XS, Xiang X, Wang X, Wang ZH, Tebbutt SJ, Kollmann TR, Fish EN. Interferon-α2b Treatment for COVID-19. Front Immunol. 2020 May 15;11:1061. doi: 10.3389/fimmu.2020.01061. Erratum in: Front Immunol. 2020 Oct 27;11:615275. PMID: 32574262; PMCID: PMC7242746.
- Aricò E, Castiello L, Bracci L, Urbani F, Lombardo F, Bacigalupo I, Ancidoni A, Vanacore N, Falcione A, Reggiani C, Dutti GM, Maglie MG, Papa O, Bartoletti PL, Ozzella G, Bevilacqua N, Nicastri E, Belardelli F, Sconocchia G. Antiviral and immunomodulatory interferon-beta in high-risk COVID-19 patients: a structured summary of a study protocol for a randomised controlled trial. Trials. 2021 Sep 3;22(1):584. doi: 10.1186/s13063-021-05367-6. PMID: 34479601; PMCID: PMC8413691.
- Arabi YM, Alothman A, Balkhy HH, Al-Dawood A, AlJohani S, Al Harbi S, Kojan S, Al Jeraisy M, Deeb AM, Assiri AM, Al-Hameed F, AlSaedi A, Mandourah Y, Almekhlafi GA, Sherbeeni NM, Elzein FE, Memon J, Taha Y, Almotairi A, Maghrabi KA, Qushmaq I, Al Bshabshe A, Kharaba A, Shalhoub S, Jose J, Fowler RA, Hayden FG, Hussein MA; And the MIRACLE trial group. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018 Jan 30;19(1):81. doi: 10.1186/s13063-017-2427-0. PMID: 29382391; PMCID: PMC5791210.
- Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020 Jun;178:104791. doi: 10.1016/j.antiviral.2020.104791. Epub 2020 Apr 7. PMID: 32275914; PMCID: PMC7138382.
- Arabi YM, Asiri AY, Assiri AM, Abdullah ML, Aljami HA, Balkhy HH, Al Jeraisy M, Mandourah Y, AlJohani S, Al Harbi S, Jokhdar HAA, Deeb AM, Memish ZA, Jose J, Ghazal S, Al Faraj S, Al Mekhlafi GA, Sherbeeni NM, Elzein FE, Hayden FG, Fowler RA, AlMutairi BM, Al-Dawood A, Alharbi NK. Heterogeneity of treatment effect of interferon-β1b and lopinavir-ritonavir in patients with Middle East respiratory syndrome by cytokine levels. Sci Rep. 2022 Oct 28;12(1):18186. doi: 10.1038/s41598-022-22742-8. PMID: 36307462; PMCID: PMC9616407.
- Alavi Darazam I, Shokouhi S, Pourhoseingholi MA, Naghibi Irvani SS, Mokhtari M, Shabani M, Amirdosara M, Torabinavid P, Golmohammadi M, Hashemi S, Azimi A, Jafarazadeh Maivan MH, Rezaei O, Zali A, Hajiesmaeili M, Shabanpour Dehbsneh H, Hoseyni Kusha A, Taleb Shoushtari M, Khalili N, Soleymaninia A, Gachkar L, Khoshkar A. Role of interferon therapy in severe COVID-19: the COVIFERON randomized controlled trial. Sci Rep. 2021 Apr 13;11(1):8059. doi: 10.1038/s41598-021-86859-y. PMID: 33850184; PMCID: PMC8044200.
- Nguyen HA, Cooke GS, Day JN, Flower B, Phuong LT, Hung TM, Dung NT, Khoa DB, Hung LM, Kestelyn E, Thwaites GE, Chau NVV; SEARCH Investigators; Turner HC. The direct-medical costs associated with interferon-based treatment for Hepatitis C in Vietnam. Wellcome Open Res. 2020 Sep 11;4:129. doi: 10.12688/wellcomeopenres.15408.2. PMID: 32734002; PMCID: PMC7372532.
- Bittner B, Richter W, Schmidt J. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs. 2018 Oct;32(5):425-440. doi: 10.1007/s40259-018-0295-0. PMID: 30043229; PMCID: PMC6182494.
- Hauschild A, Gogas H, Tarhini A, Middleton MR, Testori A, Dréno B, Kirkwood JM. Practical guidelines for the management of interferon-alpha-2b side effects in patients receiving adjuvant treatment for melanoma: expert opinion. Cancer. 2008 Mar 1;112(5):982-94. doi: 10.1002/cncr.23251. PMID: 18236459.
- Interferon Beta-1a Subcutaneous Injection: MedlinePlus Drug Information. (n.d.). March 24, 2023. https://medlineplus.gov/druginfo/meds/a604005.html
- Kim YM, Shin EC. Type I and III interferon responses in SARS-CoV-2 infection. Exp Mol Med. 2021 May;53(5):750-760. doi: 10.1038/s12276-021-00592-0. Epub 2021 May 6. PMID: 33953323; PMCID: PMC8099704.
- Zheng F, Zhou Y, Zhou Z, Ye F, Huang B, Huang Y, Ma J, Zuo Q, Tan X, Xie J, Niu P, Wang W, Xu Y, Peng F, Zhou N, Cai C, Tang W, Xiao X, Li Y, Zhou Z, Jiang Y, Xie Y, Tan W, Gong G. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: A randomized, open-label, parallel-group trial. Int J Infect Dis. 2020 Oct;99:84-91. doi: 10.1016/j.ijid.2020.07.053. Epub 2020 Aug 3. PMID: 32758689; PMCID: PMC7397938.
- WHO Solidarity Trial Consortium; Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021 Feb 11;384(6):497-511. doi: 10.1056/NEJMoa2023184. Epub 2020 Dec 2. PMID: 33264556; PMCID: PMC7727327.
- Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, Gabbay FJ, Davies DE, Holgate ST, Ho LP, Clark T, Djukanovic R, Wilkinson TMA; Inhaled Interferon Beta COVID-19 Study Group. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021 Feb;9(2):196-206. doi: 10.1016/S2213-2600(20)30511-7. Epub 2020 Nov 12. PMID: 33189161; PMCID: PMC7836724.
- Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, Kazemzadeh H, Yekaninejad MS. A Randomized Clinical Trial of the Efficacy and Safety of Interferon β-1a in Treatment of Severe COVID-19. Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01061-20. doi: 10.1128/AAC.01061-20. PMID: 32661006; PMCID: PMC7449227.
- Feld JJ, Kandel C, Biondi MJ, Kozak RA, Zahoor MA, Lemieux C, Borgia SM, Boggild AK, Powis J, McCready J, Tan DHS, Chan T, Coburn B, Kumar D, Humar A, Chan A, O'Neil B, Noureldin S, Booth J, Hong R, Smookler D, Aleyadeh W, Patel A, Barber B, Casey J, Hiebert R, Mistry H, Choong I, Hislop C, Santer DM, Lorne Tyrrell D, Glenn JS, Gehring AJ, Janssen HLA, Hansen BE. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med. 2021 May;9(5):498-510. doi: 10.1016/S2213-2600(20)30566-X. Epub 2021 Feb 5. PMID: 33556319; PMCID: PMC7906707.