More Information
Submitted: March 09, 2024 | Approved: April 15, 2024 | Published: April 16, 2024
How to cite this article: Kuunibe JK, Apiribu F, Laari TT, Atanuriba GA, Dzomeku VM, et al. Exploring the Barriers to Clinic-based Screening for Sexually Transmitted Infections (STIs) among Men in Ghana: A Qualitative Study. J Community Med Health Solut. 2024; 5: 044-051.
DOI: 10.29328/journal.jcmhs.1001046
Copyright License: © 2024 Kuunibe JK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Barriers; Sexually transmitted infections; Screening; Men
Acronyms and abbreviations: STIs: Sexually Transmitted Infections; STDs: Sexually Transmitted Diseases; PID: Pelvic Inflammatory Disease; WHO: World Health Organization; UD: Urethral Discharge; SSA: Sub-Saharan Africa; HIV: Human Immune Virus; AIDS: Acquired Immune Deficiency Syndrome; COREQ: Consolidated Criteria for Reporting Qualitative Research; CHPS: Community-based Health Planning and Services; SDGs: Sustainable Development Goals
Exploring the Barriers to Clinic-based Screening for Sexually Transmitted Infections (STIs) among Men in Ghana: A Qualitative Study
Joseph Kuufaakang Kuunibe1,2 , Felix Apiribu1, Timothy Tienbia Laari1,3
, Felix Apiribu1, Timothy Tienbia Laari1,3 *, Gideon Awenabisa Atanuriba1,4
*, Gideon Awenabisa Atanuriba1,4 , Veronica Millicent Dzomeku1, Victoria Bubunyo Bam1, Abigail Kusi-Amponsah Diji1/sup>, Adwoa Bemah Boamah Mensah, Philemon Adoliwine Amooba1, Rumana Saeed Mohammed5
, Veronica Millicent Dzomeku1, Victoria Bubunyo Bam1, Abigail Kusi-Amponsah Diji1/sup>, Adwoa Bemah Boamah Mensah, Philemon Adoliwine Amooba1, Rumana Saeed Mohammed5 and Esther Benni6
and Esther Benni6
1Department of Nursing, Faculty of Allied Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
2Midwifery Training College, Tumu, Ghana
3Presbyterian Primary Health Care, Bolgatanga, Ghana
4Department of Pediatrics, Northern Regional Hospital, Tamale, Ghana
5Department of Nursing, Garden City University College, Kumasi, Ghana
6Tumu Municipal Hospital, Tumu, Ghana
*Address for Correspondence: Timothy Tienbia Laari, Department of Nursing, Faculty of Allied Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, Email: [email protected]
The burden of sexually transmitted infections (STIs) continues to increase with over one million curable STIs occurring daily worldwide. Sex disparity in the rates of testing for STIs can compromise the efforts to reduce the incidence of STIs. The study aimed to explore the barriers to facility-based screening for STIs among men in Ghana. A qualitative exploratory design was employed in this study. Using a semi-structured interview guide, individual in-depth interviews were conducted among purposively sampled men from November 2019 to January 2020. Data saturation was achieved at the ninth participant. Audio-recorded interviews were transcribed verbatim and analysed thematically through Braun and Clarke’s approach. Four themes emerged from the analyses: (1) lack of privacy from healthcare providers, (2) mistrust of healthcare providers, (3) the burden of handling thoughts of positive status, and (4) fear of stigmatisation. These barriers deterred men from seeking clinic-based screening for STIs. The barriers were multi-factorial and a major hindrance to ensuring that people are aware of their STI status through screening and diagnosis. It is imperative to consider these barriers when designing STI screening interventions and policies to help promote facility-based screening for STIs among men in Ghana.
Sexually transmitted infections (STIs) are a major global health concern that affects almost everyone and is sometimes known as sexually transmitted diseases (STDs). They are a group of infections transmitted from one person to another through sexual contact with about 30 different types of parasitic, bacterial, and viral pathogens causing these infections. Commonly reported STIs such as; chlamydia, syphilis, chancroid, and gonorrhoea are curable while hepatitis B, human papillomavirus (HPV), genital herpes, and human immune-deficiency virus/acquired immune deficiency syndrome (HIV/AIDS) can only be managed [1-3]. STIs account for a huge burden of morbidity and mortality globally because of their effect on reproductive and child health [4], most especially in many developing countries [5]. The majority are asymptomatic leading to difficulty in early diagnosis but easy to spread and have both physical and psychological health effects such as infertility, pelvic inflammatory disease (PID), and acute illness among others. STIs are noted to facilitate the transmission of HIV/AIDS and other reproductive diseases and infections [6].
Response to this worldwide need resulted in the promulgation of the World Health Organization (WHO) plan to eradicate STIs in 2016-2021[1,3], developing guidelines for STI screening and management which largely is a syndromic approach and other cost-effective testing approaches.
Most studies that assess the barriers to screening for STIs among men are mostly involving men who have intercourse with their fellow men [7]. Globally men shun seeking routine medical screening and counselling and disregard symptoms or seek medical care late [8]. A study conducted in Australia reported that men are less likely than women to visit the healthcare facility for STI screening and are most likely to report late to the facility with a poor prognosis [9].
The burden of Sexually Transmitted Infections (STIs) continues to increase with over one million curable STIs occurring daily worldwide [10]. Evidence shows new infections of the four curable STIs globally, rose from 359 million in 2012 to 376 million in 2016 [11]. According to Muntaru, et al. [1], STDs are among the top five categories of reasons people seek medical assistance in Low- and Middle-Income Countries (LMICs). About 93 million cases of STIs are recorded in sub-Saharan Africa (SSA) alone each year according to a multi-country study [12].
A study with both men and women as participants in Ghana revealed that 66% of gonorrhoea and 58% of chlamydia cases were diagnosed in men although the men constituted only 42% of the study sample [2]. This indicates the high prevalence and incidence of gonorrhoea and chlamydia in men amidst the bad STI-seeking behaviour of men [13]. Furthermore, dysuria (50%) and urethral discharge (UD) (48%) were worryingly linked with the diagnosis of gonorrhoea rather than chlamydia in men [2].
Internationally studies on the barriers to STI screening have revealed interesting findings. In Canada, delays due to wait times, clinic distance, embarrassment with testing, discomfort discussing sexual history, fearing judgement from healthcare providers [14], non-inclusive clinic environments, and lack of provider knowledge and competency, have been cited as barriers to STI screening. In the United States of America (USA), providers’ lack of testing knowledge, lack of time, discomfort with sexual history taking and genital examinations, and patient reluctance have been cited as barriers [15]. In Australia, limited knowledge about asymptomatic STIs, attitudinal barriers, and a lack of skills to communicate about STIs have been cited as barriers to STI screening [16].
In SSA, few studies have explored the barriers to STI screening and are mostly conducted among female participants. In Kenya, lack of symptoms, fear of positive test results, stigmatization from parents and family members, and uncomfortable and embarrassing methods of specimen collection were identified as barriers to STI screening [17].
Most of the studies assessing the barriers to screening for STIs are usually conducted among people attending healthcare facilities. To this end non-patient samples are underused. Therefore, some researchers are of the view that the over-reliance on patient samples in STI screening research demonstrates a misrepresentation of the populations in need of STI care where only the characteristics, needs, and views of those attending health facilities for care are chronicled [18]. Mapp, et al. [18] further argued that STI research should extend beyond the biomedically dominated angles of infections to include the social experience of genito-urinary health care seeking using experienced symptoms as a way of recruiting participants [18].
In Ghana, data on STI prevalence exist but there is a dearth of data on the barriers to screening for STIs especially among men [13]. There is also a paucity of published data on STIs in Ghana [19]. A review of the national HIV and AIDS, STI policy of February 2013 supports this view as information about STIs is skewed at HIV/AIDS [20]. Individual, social, and cultural factors influence screening for STIs as well as the prevention strategies to adopt [21,22]. Based on this, the behaviour of individuals with STIs must be well studied to inform strategies for intervention. Behaviour change is necessary for the menace of STIs to be curtailed [21] since STIs are behaviour-based diseases [13].
Most studies on the topic in the Ghanaian context are mostly skewed toward HIV and female participants. Therefore, the study aimed to explore the barriers to clinic-based screening for STIs among men in Ghana.
Study design
A descriptive qualitative study design was employed. The choice of this design afforded the researchers to understand the significance and meaning that different participants would attach to the problem [23], and more suitable in this situation where little is known [24] about the barriers to STI screening among men. Therefore, this design was employed to describe the barriers to screening for STIs among men in response to experienced symptoms of STIs. We report this study guided by the Consolidated Criteria for Reporting Qualitative Research (COREQ) checklist (Supplementary-files 1) [25].
Research area
The Sissala East Municipality is one of the 11 Municipalities and Districts in the Upper West Region. The Municipality is located in the north-western part of Ghana in the Upper West Region and the administrative capital is Tumu. The municipality has a total population of 80,619 comprising 39,868 males and 40,751 females with over 76% being rural dwellers [26]. Communities in the Sissala East Municipality are far apart and healthcare providers travel long distances to provide health services to people [27]. The poor road network hinders the provision of health services to the people [28]. The formal health system in the Sissala East Municipal consists of one district hospital, five health centres, and some Community-based Health Planning and Services (CHPS) centres [29]. These health facilities provide STI screening and treatment services to community members. There are also private pharmacies and over-the-counter medicine seller shops within the townships. The municipal health administration also periodically carries out outreach programmes to various communities to provide consultation, screening, and treatment to the people. There is also an ongoing HIV/AIDS and other STI control programmes in the municipality due to the strategic position of the district close to the border between Ghana and Burkina Faso [29].
Study population, sampling and sample size
The population of this study was men living in the Sissala East Municipality, Upper West Region. We included all males who at the time of data collection were experiencing UD, dysuria, and/or GUD or have ever experienced any of the stated symptoms in the past and aged 18 years and older. The purposive sampling method was used to select participants who were most knowledgeable in providing the right information about the phenomenon [30]. The sample size was determined by data saturation, a point where no new theme emerged and thus information was repetitive, which was reached with the ninth participant interview.
Data collection tool and method
A semi-structured interview guide (Supplementary file 2) developed by the authors and pre-tested in Nandom with four participants was used to collect data. The interview guide contained open-ended questions with prompts such as: tell me what you know about having an STI test; What can prevent you from going for an STI test? The data were collected from November 2019 to January 2020 where participants were selected from “keep fit clubs” and “youth parliaments” in the township. These groups are predominantly made of males from within and around the town. The first author enrolled in two of the Keep Fit clubs and approached the participants face-to-face. The male gender of the first author further provided an advantage as evidence shows that people are more comfortable discussing issues of sexuality with service providers of the same gender [31-33].
Seventeen men met the inclusion criteria and interviews were conducted until the data were saturated on the ninth participant. All participants gave their written consent by signing the informed consent form after a detailed explanation of the study processes. With permission, the interviews were audio-recorded using a digital voice recorder and lasted between 30 minutes and 1 hour.
Interviews were conducted at the homes of participants (participants’ choice) and no one else was present during the interviews. The interviews were conducted by the first author, a male qualitative researcher with rich experience in clinical and community health nursing. One research assistant helped in collecting data from two participants in the Sissali language and the first author was present to observe. The data were analysed concurrently with data collection which helped the researcher to seek clarifications on the data from participants. Important gestures and non-verbal expressions were noted in a field notebook. No participant was coerced in any way during the data collection process. Audio tapes were played back to participants to verify the data and no repeat interviews were conducted.
Data analysis and management
Data were analysed using the thematic analysis method where audio recordings were transcribed verbatim and analysed by the first and second authors using NVivo version 12 software. Data that were collected in the Sissali language were transcribed in Sissali and translated to English and back to Sissali to ensure credibility. The Sissala Language Programme (SILAP), a Non-Governmental Organization (NGO), was contracted to do the transcription of the Sissali audio clips and the translation into the English Language. The interviews conducted in the English Language were transcribed verbatim by the first author. The transcripts were identified with pseudonyms and uploaded into the software and analysed according to the steps outlined by Braun and Clarke [34].
The first and second authors read through the individual transcripts severally to become familiar with the data and noted down initial ideas. Initial codes were generated independently by the authors. Statements and phrases about barriers to screening for STIs among men were identified and collated relevant to each code. Potential themes were then searched for from the list of codes. Relationships between codes and themes were identified to form the main themes. The identified themes were reviewed and some themes were collapsed. The candidate themes were then refined where the validity of individual themes was considered in relation to the data sets. The themes were then defined and further refined. What each theme represented was identified taking note of their coherence and internal consistency with the narratives. A report was then produced from the data to present the story in a concise, coherent, and logical manner within the themes. Copies of the audio clips and the transcripts were encrypted in folders in a password-protected computer of the first author and also saved on an external hard drive and flash drive to prevent data loss.
Trustworthiness/rigour
Rigour was achieved through efforts that were employed to ensure that the information represented the viewpoints of participants. Lincoln and Guba’s [35] concepts of ensuring trustworthiness were used. To achieve credibility in this study, the researchers employed iterative questioning and probes to extract related data through rephrased questions, and any untruth demonstrated by any participant was discarded [23]. Participants were made to verify the data on the spot by replaying the recordings to them to ensure that the data represented their exact opinions and not that of the researchers [36]. We achieved transferability by providing a vivid description of processes of inquiry and a clear description of the setting to allow for judgment based on the similarity of context [37]. Data transcription was done by the first author and coding was done by two researchers and validated by the other authors.
Ethical considerations
Ethical clearance was obtained from the Committee on Human Research, Publications, and Ethics (CHRPE) of the Kwame Nkrumah University of Science and Technology (KNUST) with reference number CHRPE/AP/636/19. Written permission was also obtained from the Sissala East Municipal Director of Health Services before the commencement of the data collection. All participants were given a full explanation of the study processes including their right to voluntary participation and withdrawal without penalties. All participants gave written consent to participate in the study and signed the informed consent form. To ensure anonymity and confidentiality, all personal identifying information of participants was removed and pseudonyms assigned.
Socio-demographic characteristics
Nine men participated in the study after data saturation and they were aged between 27 and 38 years with the majority (n = 5; 55.6%) of them being married. All but one of the participants were engaged by way of employment either informally (privately) or with a government institution. Of those who attained a tertiary level of education (n = 5), all of them were engaged in the public sector and those who could not go beyond Senior High School (SHS) were mostly businessmen. The majority (n = 5; 55.6%) of the participants were Sissalas. The majority of the Sissalas in Tumu are Muslims with the Christians being constituted by the other smaller ethnic groups including Dagaabas (n = 3) and Kassena (n = 1). The socio-demographic characteristics of participants are presented in Table 1.
| Table 1 : Socio-demographic characteristics of respondents. | ||||
| Participant Pseudonym | Marital Status | Educational Level | Ethnic Background | Religion |
| Musah | Married | Tertiary | Sissala | Islam |
| Kassim | Married | Senior High School | Sissala | Islam |
| Awal | Single | Senior High School | Sissala | Islam |
| Dramani | Married | Primary School | Sissala | Islam |
| Peter | Married | Tertiary | Kassena | Christian |
| Jeremiah | Single | Tertiary | Dagaaba | Christian |
| James | Single | Senior High School | Dagaaba | Christian |
| Mathias | Single | Tertiary | Dagaaba | Christian |
| David | Married | Tertiary | Sissala | Christian |
| NABCo: Nation Builders Corps | ||||
Themes and sub-themes
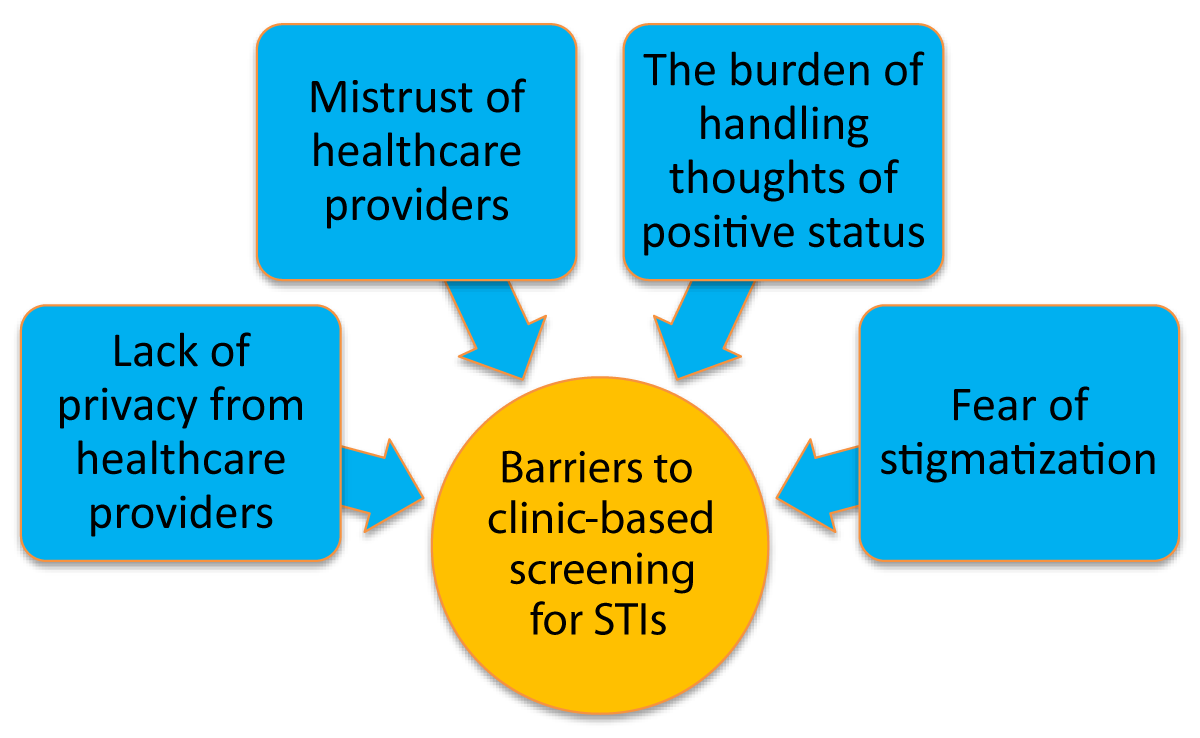
Four main themes emerged from the analysis of the data. These include; (1) lack of privacy from healthcare providers, (2) mistrust of healthcare providers, (3) the burden of handling thoughts of positive status, and (4) fear of stigmatisation. The themes are presented in Figure 1.
Figure 1: Barriers to clinic-based screening for STIs.
Lack of privacy from healthcare providers
Participants in the study mentioned that the lack of privacy from healthcare providers in the clinics discouraged them from seeking STI screening services. The men in the study were not comfortable being screened for STIs by healthcare providers who were familiar with them. They described the lack of privacy as being screened for STIs by healthcare staff who knew them. The men bemoaned the lack of privacy mainly because the town (Tumu) is small and most of the healthcare providers were indigenes of the town. Therefore, being screened for STIs by healthcare staff who are familiar to the participants was considered to be a breach of their privacy. This consequently deterred them from seeking STI screening services from the clinics.
“Sometimes, you look at the community in which you are. Look at Tumu, for instance, we are in Tumu here, and then let’s say, almost 60 to 70% of the health workers there are from here. We know ourselves. And then imagine you go to such a place [clinic] to go and test for STI and you are to be attended to by the nurses you know. So because of that I don’t feel comfortable going them with my STI” (Awal, Single).
“…and because I know most of the nurses in the health facility, it is difficult for me to go there and ask them to test me for STI. This community is small so I know all the workers there and they also know me. So it is just because of privacy” (Peter, Married).
Another participant narrated that he previously sought STI screening from the clinic but indicated that he will not patronise the clinic anymore because of too many familiar staff in the clinic. He had this to say:
“As for me I have stopped going to the clinic to test for my STI. Even when I am experiencing the symptoms I will not go because if I go there the workers who are going to test me are all people I know” (Kassim, Married).
Mistrust of healthcare providers
Another theme generated from the analysis of the data is mistrust of healthcare providers. The participants of the study did not trust the healthcare providers with their STI history hence, they did not seek STI screening services from health facilities. The participants indicated that the staff in the health facilities are too unprofessional to confide in their sensitive STI information. Participants indicated that the inability of some healthcare staff to keep sensitive information was the reason for the mistrust. The men in the study were particularly concerned about the possibility of the healthcare providers leaking their STI status to their friends. Other men feared that the healthcare providers would tell their wives about their condition. Owing to these issues of mistrust, the men declined to go for SCI screening in the health facility. Two of the participants had this say about the mistrust of healthcare providers:
“…and also I don’t have trust in the health workers in the facility because some of them cannot keep secrets. So they can tell their friends and even others about the results of the test. I don’t trust them with that” (James, Single).
“I don’t want other people to know if I am having any STI in my body but as for the hospital, if I go there, maybe the one who will do the test can tell his friends about it. Me too I am not comfortable with that so I don’t even go there in the first place” (Dramani, Married).
Another participant explained that the health staff cannot keep their test results secret hence, they did not go to the health centre for STI screening.
“Because sometimes there are some health staff in some health centres that when you go in for the test they may not keep that secret…so the trust is not there” (Mathias, Single).
A participant narrated that he did not patronise health facilities for STI screening because he felt that the healthcare providers would tell his wife about his results. This is what he:
“For me it is all about the mistrust. Mistrust in the sense that I don’t even want the partner to know if I have STI, but you know when it’s the hospital [Medical Centre] once you have it the health staff can tell my wife. Meanwhile, I don’t also want my wife to know about it so I don’t go there for testing” (Musah, Married).
The burden of handling thoughts of positive status
Participants described the burden of handling thoughts of positive status as enormous and constituted a barrier to them seeking STI screening. They believed that the psychological effect of a positive STI test is more burdensome than the infection itself. Participants described the experience of having a positive STI test result as scary and handling the thoughts as challenging. Due to the inability to handle the thoughts of positive STI test results, the men deemed it better not to know their STI status by avoiding clinic-based screening for their STIs. The reason for fear is attributed to the feeling that some STIs cannot be treated. A participant asserted:
“It’s very scary. Well, as earlier stated, getting to know that you are positive is equally a very challenging thing to deal with because it is something you cannot just come home and just sleep over it. Because if today I come to the facility and you tell me that ooh my brother you have syphilis, I don’t know how I’m going to manage this syphilis or how it is going to finish within the shortest possible time, so in itself, it’s going to be a burden” (David, Married).
Other participants narrated that the psychological effect of knowing their status is difficult to deal with.
“It is not because something is preventing me but the mind-set. I feel that I’m very confident and I’m not infected to walk to the health centre to screen for STI but my mind-set is that once I test and I realize that I am positive…my mind-set alone, it will drag me backwards. That is one of the reasons why I don’t even want to go to the health centre for those kinds of tests” (Kasim, Married).
“Well, as for me, getting to know that am positive for an STI is equally a very difficult thing to deal with because it is something that I cannot just erase from my mind and thoughts. So to avoid this burden on my thoughts, I don’t do it [screen for STI]” (Jeremiah, Single).
Fear of stigmatisation
Fear of stigmatisation was another theme that emerged from the analysis of the data. Stigma was defined by participants as the act of people pointing fingers at them in public because of their STI status. The participants did not seek STI screening from health facilities because of the fear of stigma. Some of the participants feared that if their positive STI status was known by community members, they would be stigmatised in the community. The men in the study feared that members of the town would point fingers at them should their positive STI test result turn out to be positive. To avoid finger-pointing (stigma), they simply avoided seeking STI screening at the health facility. Some participants had this to say about perceived stigma.
“…and after the test, if the test results is made public, people will be pointing hands [fingers] at me that this guy has this sickness [STI]…so because of the fear of stigma I don’t want to go to the health centre for STI tests” (James, Single).
“…if that happens, anytime I am moving about in town they [people] will say ooh this is the man who is infected with this disease. So this subdues [prevents] me and other people from going in for STI test in the hospital” (Jeremiah, Single).
Some of the participants have seen other people stigmatised in their communities. Therefore, they did not want to be treated the same way. They described stigma as unfortunate and did not want to be put in that situation by others. Another participant had this to say about perceived stigma.
The objective of our study was to explore the barriers to screening for STIs among men in Ghana. We report the findings from nine men who have ever experienced UD, GUD, and/or dysuria or were experiencing these symptoms at the time of the interviews. They were aged between 27 and 38 years. This age range falls within the age range of participants for similar studies in New Zealand [38] and South Korea [39]. The majority (n = 5) of the participants were married which signifies that STIs do not only affect single men who are more likely to have multiple sexual partners. Similar findings were reported in a study in the Ashanti region of Ghana [13] where the majority of the participants with STIs were either married (n = 4: 33%) or recently divorced (n = 5: 41.7%). However, contrary findings in South Africa [40] indicate that fewer married men reported STIs and suggested this could be attributed to the fact that married men may have just one sexual partner. The majority (5) of the participants were Christians and four were of the Islamic religion. Although previous studies have reported religion as a barrier to STI screening [41,42], this did not emerge as a theme in the current study.
Our study found that the lack of privacy from healthcare providers and mistrust of healthcare providers were barriers to clinic-based screening for STIs among men. Privacy and trust of healthcare providers are important factors for the uptake of STI screening services. However, due to the mutual familiarity between the men in our study and the healthcare providers, the men were not comfortable going to the health facility for STI screening. The participants also feared that some healthcare staff could tell others about the positive STI results. These deterred them from seeking screening for STIs in health facilities. These findings are dissimilar to the findings of a study in New Zealand where familiarity with healthcare providers was not a matter of concern for participants who sought STI screening services [32]. This contrasting finding could be due to the cultural differences in the settings of the studies. However, our findings are consistent with the findings of a study in South Africa [43] and two systematic reviews [31,44]. This similarity could be because most of the studies in these reviews were from LMICs where people live in smaller communities and members are familiar with each other. In most Ghanaian communities, people feel embarrassed and uncomfortable when screened for STIs by familiar healthcare staff and would rather prefer to be attended to by unfamiliar staff as reported in Australia [9] and South Africa [40]. Men may worry about their STI status being disclosed to others, especially if they live in small communities or have close ties with their healthcare providers.
This implies that the men had qualms with entrusting the healthcare workers with information about their STIs, mainly because they feared such sensitive information could be leaked. These findings raise concerns about the ethical and professional conduct of the healthcare staff. Mistrust of healthcare providers and the lack of privacy can create significant barriers to STI screening by reducing patients’ willingness to discuss their sexual health history with the healthcare staff and seek appropriate care. Therefore, healthcare providers need to maintain the privacy of all patients to protect sensitive health information and maintain their trust. This can be attained by training healthcare staff on trust and privacy policies and procedures. Health facility managers should ensure that all staff receive comprehensive training on trust and privacy policies and procedures. This includes training on handling patient records, using secure communication channels, and following privacy regulations.
The current study found that the burden of handling thoughts of positive status was another barrier to seeking screening for STI by men in our study. This finding concurs with the findings of earlier studies in Uganda [8] and Australia [45]. The psychological effect of a positive STI test result was of great concern to participants. The burden of handling the thoughts of positive STI results was described to be more burdensome than the infection itself. This is because some of the participants erroneously thought all STIs were not treatable. This finding highlights a knowledge gap among the participants regarding the treatment of STIs. Therefore, we recommend that health education interventions be implemented and targeted at increasing knowledge on the types of STIs and how they can be treated or managed to ease the psychological burden associated with positive STI test results.
The findings of our study revealed that the fear of stigmatisation was a barrier to seeking clinic-based screening for STIs among men. This finding concords with the findings of earlier studies in Kenya [17] and the USA [41,46]. Generally, men seem to be more concerned about being stigmatized if they are seen seeking screening for STIs from health facilities as similar findings were reported [9,32,40]. The fear of stigmatisation surrounding STI screening can have a significant impact on men’s willingness to get screened and seek treatment. It is essential for healthcare providers to create a safe and non-judgmental environment that respects patients’ privacy and confidentiality to help overcome the fear of being stigmatised. This can be achieved by training healthcare providers to use inclusive, non-judgmental language when communicating with patients and avoid judgmental comments related to a patient’s lifestyle, appearance, or health condition. This creates an environment where patients feel heard, respected, and their fears allayed.
Our study is limited to the extent that it relied on participants’ understanding of STI symptoms. Also, there was a possibility of selection bias as the researchers only recruited participants at “keep fit clubs” and “youth parliaments” in the township. Again, the study is liable to recall bias. A strength of our study is that it is the first study in Ghana to use a community approach to explore such a phenomenon among men. To this end, the study findings are crucial to improving clinic-based screening for STIs among men in Ghana.
The study revealed that multi-factorial barriers such as; lack of privacy from healthcare providers, mistrust of healthcare providers, burden of handling thoughts of positive status, and fear of stigmatisation deterred men from seeking clinic-based screening for STIs. These multi-factorial barriers could derail the achievement of goal three of the Sustainable Development Goals (SDGs). The impact of these barriers to clinical practice is far-reaching and requires proactive health sensitization among the public and training of healthcare providers to provide culturally sensitive STI screening services. It is imperative to consider these barriers when designing STI screening interventions and policies in Ghana.
The authors are grateful to the participants for opening up and consenting to participate in the study despite the sensitive and ego-threatening nature of the subject.
Data availability
The transcripts used for the analysis of the study are available from the corresponding author upon reasonable request.
Author contributions
Conceptualization: Kuunibe JK, Apiribu F; Data curation: Laari TT, Adwoa BBM; Formal analysis: Kuunibe JK, Apiribu F, Laari TT; Methodology: Kuunibe JK, Apiribu, F, Atanuriba GA; Resources: Kuunibe JK; Validation: Apiribu F, Dzomeku VM, Amooba PA, Diji AK; Writing - original draft: Kuunibe JK, Laari TT, Apiribu F; Writing - review & editing: Bam VB, Benni E, Mohammed RS.
- Mutaru A-M, Asumah M, Ibrahim M, Sumaila I, Hallidu M, Mbemah J. Knowledge on Sexually Transmitted Infections (STIs) and sexual practices among Nursing Trainees in Yendi Municipality, Northern Region of Ghana. European Journal of Health Sciences. 2021; 6(4):33–47.
- Dela H, Attram N, Behene E, Kumordjie S, Addo KK, Nyarko EO, Kyei NNA, Carroll JNA, Kwakye C, Duplessis CA, Adams N, Garges E, Letizia AG. Risk factors associated with gonorrhea and chlamydia transmission in selected health facilities in Ghana. BMC Infect Dis. 2019 May 16;19(1):425. doi: 10.1186/s12879-019-4035-y. PMID: 31096920; PMCID: PMC6524331.
- Abdurahman C, Oljira L, Hailu S, Mengesha MM. Sexual and reproductive health services utilization and associated factors among adolescents attending secondary schools. Reprod Health. 2022 Jul 15;19(1):161. doi: 10.1186/s12978-022-01468-w. PMID: 35840973; PMCID: PMC9287868.
- Risenga PR, Davhana-Maselesele M. A concept analysis of young adults; Perception of HIV Counselling and Testing. Health SA Gesondheid. 2017; 22:213-20.
- Ramjee G, Abbai NS, Naidoo S. Women and Sexually Transmitted Infections in Africa. Open Journal of Obstetrics and Gynecology. 2015; 5(July):385–99.
- Kassie BA, Yenus H, Berhe R, Kassahun EA. Prevalence of sexually transmitted infections and associated factors among the University of Gondar students, Northwest Ethiopia: a cross-sectional study. Reprod Health. 2019 Nov 8;16(1):163. doi: 10.1186/s12978-019-0815-5. PMID: 31703688; PMCID: PMC6842222.
- Veronese V, van Gemert C, Bulu S, Kwarteng T, Bergeri I, Badman S, Vella A, Stoové M. Sexually transmitted infections among transgender people and men who have sex with men in Port Vila, Vanuatu. Western Pac Surveill Response J. 2015 Mar 5;6(1):55-9. doi: 10.2471/WPSAR.2014.5.1.001. PMID: 25960923; PMCID: PMC4410108.
- Lubega GN, Musinguzi B, Omiel P, Tumuhe JL. Determinants of health seeking behaviour among men in Luwero District. Journal of Educational Research and Behavioural Sciences. 2015; 4(2):37–54.
- Su JY, Belton S, Ryder N. Why are men less tested for sexually transmitted infections in remote Australian Indigenous communities? A mixed-methods study. Cult Health Sex. 2016 Oct;18(10):1150-64. doi: 10.1080/13691058.2016.1175028. Epub 2016 May 4. PMID: 27142316.
- World Health Organization. Report on global sexually transmitted infection surveillance [Internet]. Geneva, Switzerland; 2018. https://apps.who.int/iris/rest/bitstreams/1170799/retrieve
- Seidu AA, Agbaglo E, Dadzie LK, Tetteh JK, Ahinkorah BO. Self-reported sexually transmitted infections among sexually active men in Ghana. BMC Public Health. 2021 May 26;21(1):993. doi: 10.1186/s12889-021-11030-1. PMID: 34039317; PMCID: PMC8157633.
- Seidu AA, Ahinkorah BO, Dadzie LK, Tetteh JK, Agbaglo E, Okyere J, Salihu T, Oteng KF, Bugase E, Osei SA, Hagan JE Jr, Schack T. A multi-country cross-sectional study of self-reported sexually transmitted infections among sexually active men in sub-Saharan Africa. BMC Public Health. 2020 Dec 7;20(1):1884. doi: 10.1186/s12889-020-09996-5. PMID: 33287785; PMCID: PMC7722450.
- Azu MN, Richter S, Aniteye P. Ghanaian Men Living with Sexual Transmitted Infections: Knowledge and Impact on Treatment Seeking Behaviour- A Qualitative Study. Afr J Reprod Health. 2018 Sep;22(3):24-32. doi: 10.29063/ajrh2018/v22i3.3. PMID: 30381929.
- Gilbert M, Thomson K, Salway T, Haag D, Grennan T, Fairley CK, Buchner C, Krajden M, Kendall P, Shoveller J, Ogilvie G. Differences in experiences of barriers to STI testing between clients of the internet-based diagnostic testing service GetCheckedOnline.com and an STI clinic in Vancouver, Canada. Sex Transm Infect. 2019 Mar;95(2):151-156. doi: 10.1136/sextrans-2017-053325. Epub 2018 Feb 7. PMID: 29437984; PMCID: PMC6580770.
- Barbee LA, Dhanireddy S, Tat SA, Marrazzo JM. Barriers to Bacterial Sexually Transmitted Infection Testing of HIV-Infected Men Who Have Sex With Men Engaged in HIV Primary Care. Sex Transm Dis. 2015 Oct;42(10):590-4. doi: 10.1097/OLQ.0000000000000320. PMID: 26372931; PMCID: PMC4576720.
- Bell S, Aggleton P, Ward J, Murray W, Silver B, Lockyer A, Ferguson T, Fairley CK, Whiley D, Ryder N, Donovan B, Guy R, Kaldor J, Maher L. Young Aboriginal people's engagement with STI testing in the Northern Territory, Australia. BMC Public Health. 2020 Apr 6;20(1):459. doi: 10.1186/s12889-020-08565-0. PMID: 32252712; PMCID: PMC7137447.
- Avuvika E, Masese LN, Wanje G, Wanyonyi J, Nyaribo B, Omoni G, Baghazal A, McClelland RS. Barriers and Facilitators of Screening for Sexually Transmitted Infections in Adolescent Girls and Young Women in Mombasa, Kenya: A Qualitative Study. PLoS One. 2017 Jan 3;12(1):e0169388. doi: 10.1371/journal.pone.0169388. PMID: 28046104; PMCID: PMC5207488.
- Mapp F, Wellings K, Hickson F, Mercer CH. Understanding sexual healthcare seeking behaviour: why a broader research perspective is needed. BMC Health Serv Res. 2017 Jul 6;17(1):462. doi: 10.1186/s12913-017-2420-z. PMID: 28683744; PMCID: PMC5501268.
- Nyarko C, Unson C, Koduah M, Nyarko P, Galley J. Risk Factors Of Sexually-Transmitted Infections (STIs) Among Men And Women In A Mining Community In Western Ghana : A Study Of Lifetime Occurrence. International Journal of Scientific & Technology Research. 2014; 3(12).
- Ghana AIDS Commission. National HIV and AIDS, STI Policy [Internet]. Accra, Ghana; 2013. https://www.healthpolicyproject.com/pubs/153_Policyfinal.pdf
- Ali Abdulai M, Baiden F, Afari-Asiedu S, Gyabaa-Febir L, Adjei KK, Mahama E, Tawiah-Agyemang C, Newton SK, Asante KP, Owusu-Agyei S. The Risk of Sexually Transmitted Infection and Its Influence on Condom Use among Pregnant Women in the Kintampo North Municipality of Ghana. J Sex Transm Dis. 2017;2017:8642685. doi: 10.1155/2017/8642685. Epub 2017 Jan 26. PMID: 28246570; PMCID: PMC5299183.
- WHO. Standard protocol to assess the prevalence of gonorrhoea and chlamydia among pregnant women in antenatal care clinics. Geneva, Switzerland; 2018.
- Yilmaz K. Comparison of Quantitative and Qualitative Research Traditions : epistemological, theoretical, and methodological differences. European Journal of Education. 2013; 48(2):311–25.
- Mitchell ML, Jolley JM. Research Design Explained. 7th ed. USA: Wadsworth, Cengage Learning; 2010.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007 Dec;19(6):349-57. doi: 10.1093/intqhc/mzm042. Epub 2007 Sep 14. PMID: 17872937.
- Ghana Statistical Service. Ghana 2021 Population and Housing Census: Population of Regions and Districts [Internet]. Accra, Ghana; 2021. www.statsghana.gov.gh
- Ofosu W. Upper West Regional Health Services: 2016 Annual Report. Wa; 2017.
- Yahaya A. Transportation Network in the Sissala East District of Ghana : A Bane to Maternal Health. 2014.
- Ghana Districts. A repository of all Local Assemblies in Ghana: Sisala East. 2022. 1–9. https://ghanadistricts.com/Home/LinkDataDistrict/2508
- Ingham-Broomfield R. A Guide to Qualitative Research. Australian Journal of Advanced Nursing. 2015; 32(3):34–40.
- Teo CH, Ng CJ, Booth A, White A. Barriers and facilitators to health screening in men: A systematic review. Soc Sci Med. 2016 Sep;165:168-176. doi: 10.1016/j.socscimed.2016.07.023. Epub 2016 Aug 1. PMID: 27511617.
- Denison HJ, Bromhead C, Grainger R, Dennison EM, Jutel A. Barriers to sexually transmitted infection testing in New Zealand: a qualitative study. Aust N Z J Public Health. 2017 Aug;41(4):432-437. doi: 10.1111/1753-6405.12680. Epub 2017 Jun 29. PMID: 28664644; PMCID: PMC5564490.
- Mapp F, Hickson F, Mercer CH, Wellings K. How social representations of sexually transmitted infections influence experiences of genito-urinary symptoms and care-seeking in Britain: mixed methods study protocol. BMC Public Health. 2016 Jul 11;16:548. doi: 10.1186/s12889-016-3261-0. PMID: 27400780; PMCID: PMC4940703.
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology [Internet]. 2006;3(2):77–101. https://doi.org/10.1191/1478088706qp063oa
- Lincoln YS, Guba EG. Naturalistic Inquiry. Newbury Park: SAGE Publications, Inc.; 1985.
- Squires A, Dorsen C. Qualitative Research in Nursing and Health Professions Regulation. Journal of Nursing Regulation [Internet]. 2018;9(3):15–26. https://doi.org/10.1016/S2155-8256(18)30150-9
- Polit DF, Beck CT. Nursing Research: Principles and Methods. 7th ed. New York: Lippincott Williams & Wilkins; 2003. 301.
- Denison HJ. Healthcare-seeking behaviour for sexually transmitted infection testing in New Zealand : A mixed methods study. Victoria University of Wellington; 2017.
- Jayapalan S. Healthcare-seeking preferences of patients with sexually transmitted infection attending a tertiary care center in South Kerala. Indian J Sex Transm Dis AIDS. 2016 Jul-Dec;37(2):157-161. doi: 10.4103/0253-7184.188483. PMID: 27890950; PMCID: PMC5111301.
- Nyalela M. Healh Seeking Behaviour in Men Presenting with Sexually Transmitted Infections at Prince Mshiyeni Gateway Clinic and KwaMashu Community Health Centre in 2015. University of KwaZulu-Natal; 2015.
- Lichtenstein B. Stigma as a barrier to treatment of sexually transmitted infection in the American deep south: issues of race, gender and poverty. Soc Sci Med. 2003 Dec;57(12):2435-45. doi: 10.1016/j.socscimed.2003.08.002. PMID: 14572849.
- Medved Kendrick H. Are religion and spirituality barriers or facilitators to treatment for HIV: a systematic review of the literature. AIDS Care. 2017 Jan;29(1):1-13. doi: 10.1080/09540121.2016.1201196. Epub 2016 Jul 13. PMID: 27410058; PMCID: PMC9670680.
- Nyalela M, Dlungwane T, Taylor M, Nkwanyana N. Health seeking and sexual behaviour of men presenting with sexually transmitted infections in two primary health care clinics in Durban. Southern African Journal of Infectious Diseases. 2018; 0(0):1–6.
- Nagarkar A, Mhaskar P. A systematic review on the prevalence and utilization of health care services for reproductive tract infections/sexually transmitted infections: Evidence from India. Indian J Sex Transm Dis AIDS. 2015 Jan-Jun;36(1):18-25. doi: 10.4103/0253-7184.156690. PMID: 26392649; PMCID: PMC4555893.
- Hengel B, Guy R, Garton L, Ward J, Rumbold A, Taylor-Thomson D, Silver B, McGregor S, Dyda A, Knox J, Kaldor J, Maher L. Barriers and facilitators of sexually transmissible infection testing in remote Australian Aboriginal communities: results from the Sexually Transmitted Infections in Remote Communities, Improved and Enhanced Primary Health Care (STRIVE) Study. Sex Health. 2015 Mar;12(1):4-12. doi: 10.1071/SH14080. PMID: 25426563.
- Morris JL, Lippman SA, Philip S, Bernstein K, Neilands TB, Lightfoot M. Sexually transmitted infection related stigma and shame among African American male youth: implications for testing practices, partner notification, and treatment. AIDS Patient Care STDS. 2014 Sep;28(9):499-506. doi: 10.1089/apc.2013.0316. PMID: 25133501; PMCID: PMC4135319.