More Information
Submitted: May 30, 2025 | Approved: June 11, 2025 | Published: June 12, 2025
How to cite this article: Ameur O, Zahid H, Elkochri S, Tagajdid MR, Elannaz H, Hassine S, et al. Aplastic Anemia Induced by Parvovirus B19 Infection in an Immunocompetent Adult: A Case Report and Literature Review. J Community Med Health Solut. 2025; 6(1): 050-052.
Available from:
https://dx.doi.org/10.29328/journal.jcmhs.1001058
DOI: 10.29328/journal.jcmhs.1001058
Copyright license: © 2025 Ameur O, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Aplastic Anemia Induced by Parvovirus B19 Infection in an Immunocompetent Adult: A Case Report and Literature Review
Ameur O1, Zahid H2, Elkochri S1, Tagajdid MR1, Elannaz H1, Hassine S1, Chanhih N1, Ouannass S1, Reggad A1, Elqatni M1, Kasmi Z1, Laraqui A1, Touil N1, B Machichi1, Elouennass M1, Ennibi K1, Lahlou Amine I1 and Rachid Abi1*
1Virology Laboratory, Biomedical and Epidemiology Research Unit, Department of Virology, Mohammed V Military Teaching Hospital and Mohammed V University, Morocco
2Hematology Laboratory, Mohammed V Military Teaching Hospital and Mohammed V University, Morocco
*Address for Correspondence: Rachid Abi, Virology Laboratory, Biomedical and Epidemiology Research Unit, Department of Virology, Mohammed V Military Teaching Hospital and Mohammed V University, Morocco, Email: [email protected]
A 24-year-old male presented with epistaxis and gingival bleeding, physical examination revealed pallor and purpuric spots on the left upper limb. Laboratory findings showed pancytopenia. As part of the etiological workup for pancytopenia, a bone marrow aspirate was performed, revealing moderately cellular marrow with rare megakaryocytes and intranuclear inclusions in proerythroblasts. Parvovirus B19 testing in the bone marrow was conducted via nucleic acid extraction followed by PCR.
Parvovirus B19 is classified within the genus Erythroparvovirus due to its specific tropism for erythroid precursor cells in the bone marrow.
Discovered in 1975 during screening for Hepatitis B Surface antigen (HBsAg) in blood donor sera (the name B19 corresponds to the serum’s identity on the analysis plate), it was recognized in 1981 as the primary agent of acute erythroblastopenia crises in patients with constitutional red blood cell abnormalities. In 1983, it was identified as the cause of erythema infectiosum (or fifth disease in children). Its pathogenesis was later demonstrated in fetal hydrops following maternal-fetal transmission, articular manifestations, and chronic anemias of central origin in immunocompromised patients. Currently, three distinct genotypes are recognized, derived from a common ancestor during the 19th century. Genotype 1 predominantly circulates in Western countries. This variability primarily poses challenges for molecular diagnosis; no differences in replication capacity or pathogenicity have been demonstrated. We report a case of severe aplastic anemia in a 24-year-old immunocompetent adult infected with Parvovirus B19.
A 24-year-old male with no significant medical history presented with epistaxis and gingival bleeding. Physical examination revealed pallor and purpuric spots on the left upper limb.
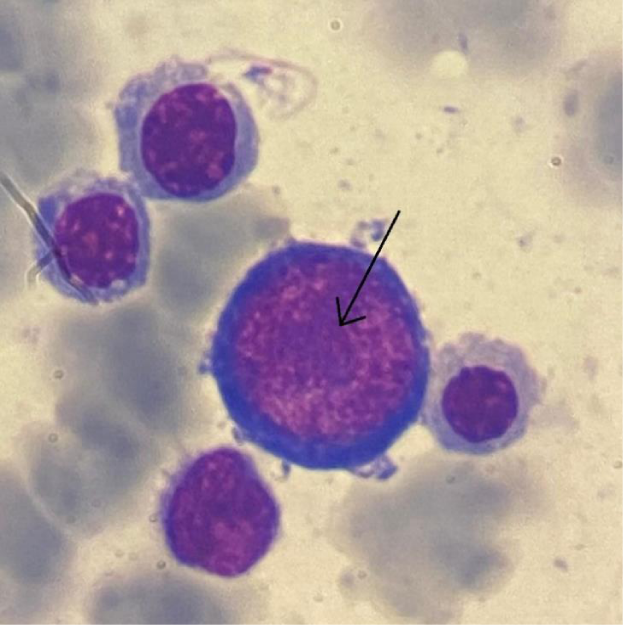
Laboratory findings showed pancytopenia with macrocytic regenerative anemia (Hemoglobin = 7.5 g/dL, platelets = 30,000/µL). As part of the etiological workup for pancytopenia, a bone marrow aspirate was performed, revealing moderately cellular marrow with rare megakaryocytes and intranuclear inclusions in proerythroblasts (Figure 1). Parvovirus B19 testing in the bone marrow was conducted via nucleic acid extraction followed by PCR using the ELITe MGB® Parvovirus B19 Kit on the ELITe InGenius system, which returned positive. Immunological markers were assessed via chemiluminescence (CLIA) on the Virclia Lotus platform using Parvovirus Virclia® IgG and IgM Monotests, confirming recent infection (IgM positive, IgG negative).
Figure 1: Bone marrow aspirate (MGG stain; x100) showing a proerythroblast with intranuclear inclusion.
Serologies for other viruses (cytomegalovirus, Epstein-Barr virus) indicated past exposure, while hepatitis B and HIV serologies were negative.
The patient’s condition worsened, with severe anemia (Hb = 5.5 g/dL) and thrombocytopenia (platelets = 8,000/µL). A bone marrow biopsy confirmed aplastic marrow.
The patient received multiple red blood cell transfusions and was treated with eltrombopag, Anti-Lymphocyte Serum (ALS), and cyclosporine without improvement. Close monitoring and supportive care led to slight clinical improvement. A bone marrow transplant was scheduled pending the identification of an HLA-matched donor.
Parvovirus B19 (B19V) is a human-specific single-stranded DNA virus with humans as its reservoir. Transmission occurs mainly through respiratory droplets but may also occur transplacentally, via blood transfusion, or organ transplantation. Clinically, B19V is associated with diverse manifestations, ranging from asymptomatic infection to severe hematological complications.
Although its primary target is erythroid precursors (via the P-antigen receptor), it can rarely induce severe Aplastic Anemia (AA) even in immunocompetent individuals without prior hematological disorders [1,2].
The pathophysiology remains unclear, but hypotheses include direct cytotoxicity of viral proteins leading to marrow destruction. However, why most healthy individuals clear the virus without developing AA while a few do remains unknown [1].
The most plausible explanation involves autoimmune mechanisms via molecular mimicry, leading to marrow cell destruction. Case reports describe remission of pancytopenia and marrow aplasia with immunosuppressive therapy, supporting this hypothesis [2,3]. However, other cases show minimal improvement with immunosuppression but successful remission after HLA-matched bone marrow transplantation, suggesting distinct mechanisms depending on patient factors [4,5].
Reported cases share common features: young adults (22 - 33 years) without prior hematological or immunological disorders [1-3], atypical presentation (absence of erythema infectiosum in 60% of cases [2,6]), severe pancytopenia (Hb < 8 g/dL, neutrophils < 500/μL, platelets < 20,000/μL), and hypocellular marrow [1,7].
The nonspecific clinical and laboratory presentation underscores the importance of molecular techniques (PCR) for B19V detection in unexplained cytopenias, even in the absence of overt infectious symptoms [1,6]. Viral DNA may persist for months, particularly in the marrow.
For PCR, nucleic acid extraction is performed on EDTA-treated whole blood (peripheral or bone marrow). Samples must be transported/stored at +2 to +8 °C for ≤3 Days or frozen at -20 °C (for up to 30 days) or -70 °C (long-term). Aliquoting is recommended to avoid freeze-thaw cycles.
The sensitivity and specificity of the ELITe MGB® kit (Figure 2) are 100%, confirmed by testing 30 B19V DNA-positive blood samples.
Figure 2: ELITe MGB® Parvovirus B19 Kit – A real-time PCR assay designed for the detection and quantification of B19V DNA. This CE-IVD-validated test is compatible with the fully automated ELITe InGenius® system.
PCR kits like ELITe MGB®, though effective for detecting B19V by targeting conserved genomic regions, may fail to detect rare variants (genotypes 2/3) or mutations in target sequences. These shortcomings justify the use of genomic sequencing, which confirms viral genotype and identifies virulence-associated mutations [8]. It serves a dual purpose:
- Diagnostic: resolving false negatives due to mutations.
- Therapeutic/research: understanding infection mechanisms and developing antivirals for persistent infections or unusual resistance to immunoglobulin [8].
Serology (IgM/IgG detection) remains valuable, particularly if PCR is unavailable. Combining serology + PCR differentiates acute infection (IgM+/PCR+) from viral persistence (IgM-/PCR+).
Therapeutically, transfusions are critical for severe cytopenias [1,7]. Immunosuppressants (ALS, cyclosporine) have induced remission in some cases [9]. However, Hematopoietic Stem Cell Transplantation (HSCT) remains the definitive treatment for refractory cases, with reported success [1].
- Rajput R, Sehgal A, Jain D, Sen R, Gupta A. Acute Parvovirus B19 Infection Leading to Severe Aplastic Anemia in a Previously Healthy Adult Female. Indian J Hematol Blood Transfus. 2012;28(2):123–6. Available from: https://doi.org/10.1007/s12288-011-0112-0
- Osaki M, Matsubara K, Iwasaki T, Kurata T, Nigami H, Harigaya H, et al. Severe aplastic anemia associated with human parvovirus B19 infection in a patient without underlying disease. Ann Hematol. 1999;78(2):83–6. Available from: https://doi.org/10.1007/s002770050477
- Al-Abdwani RM, Khamis FA, Balkhair A, Sacharia M, Wali YA. A child with human parvovirus B19 infection induced aplastic anemia and acute hepatitis: effectiveness of immunosuppressive therapy. Pediatr Hematol Oncol. 2008;25(7):699–703. Available from: https://doi.org/10.1080/08880010802341724
- Yetgin S, Cetin M, Ozyürek E, Aslan D, Uçkan D. Parvovirus B19 infection associated with severe aplastic anemia in an immunocompetent patient. Pediatr Hematol Oncol. 2004;21(3):223–6. Available from: https://doi.org/10.1080/08880010490276935
- Kaptan K, Beyan C, Ural AU, Ustün C, Cetin T, Avcu F, et al. Successful treatment of severe aplastic anemia associated with human parvovirus B19 and Epstein-Barr virus in a healthy subject with allo-BMT. Am J Hematol. 2001;67(4):252–5. Available from: https://doi.org/10.1002/ajh.1125
- Bhattarai AM, Dhakal B, Rokaya P, Karki A, Gurung S, Baral S. Aplastic anemia induced by human parvovirus B19 infection in an immunocompetent adult male without prior hematological disorders: A case report. Ann Med Surg. 2022;79:103998. Available from: https://doi.org/10.1016/j.amsu.2022.103998
- Kobayashi Y, Hatta Y, Ishiwatari Y, Kanno H, Takei M. Human parvovirus B19-induced aplastic crisis in an adult patient with hereditary spherocytosis: a case report and review of the literature. BMC Res Notes. 2014;7(1):137. Available from: https://doi.org/10.1186/1756-0500-7-137
- Seetha D, Pillai HR, Nori SRC, Kalpathodi SG, Thulasi VP, Nair RR. Molecular-genetic characterization of human parvovirus B19 prevalent in Kerala State, India. Virol J. 2021;18:96. Available from: https://doi.org/10.1186/s12985-021-01569-1
- Kaptan K, Beyan C, Ural AU, Üstün C, Çetin T, Avcu F, et al. Successful treatment of severe aplastic anemia associated with human parvovirus B19 and Epstein-Barr virus in a healthy subject with allo-BMT. Am J Hematol. 2001;67(4):252–5. Available from: https://doi.org/10.1002/ajh.1125